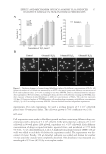
JOURNAL OF COSMETIC SCIENCE 52 alopecia (31). This is supported by the fact that men genetically defi cient in type II 5-α-reductase do not present hair loss (8). On the one hand, Finasteride, a selective in- hibitor of type II 5-α-reductase, slows the progression of baldness in a majority of men (8,32) and also in some women (33). On the other hand, dutasteride, a dual inhibitor of type I and type II 5-α-reductase activities revealed to be even more potent than fi naste- ride in improving hair growth in balding men (although not approved by the FDA for this indication), suggesting there might be an advantage in inhibiting both isozymes for this condition (31). These results suggest that extracts enriched in biochanin A, by inhib- iting both type I and type II 5-α-reductase activity in the scalp, may fi nd application in male pattern alopecia management. STIMULATION OF ECM PROTEIN SYNTHESIS The size of a hair follicle is thought to be determined by the volume of its dermal papilla, which depends on the number of cells and on the volume of the ECM (3). Regulation of the size of dermal papilla is a dynamic process involving hormonal infl uence and complex cell–matrix interactions. ECM proteins expressed in the hair follicle include collagens I, III, and VII, as well as laminins. Collagens I and III. Type I and III collagens are the most abundant types of collagen in the skin. Not surprisingly, they are also found in the hair follicle where they have the par- ticularity of presenting an unusual high ratio of collagen III/I. Collagen III is a fi brillar protein with elastic properties it is tempting to speculate that these attributes might be of particular importance for hair follicle development and maintenance. Collagen I and III are produced in the human dermal papilla throughout the hair cycle (34) and are also major constituents of the connective tissue sheet of the hair follicle (4). Laminins. Laminins are a family of large glycoproteins comprising more than 50 members that are the major constituents of basement membranes. They display a remarkable Figure 3. Inhibition of type I and type II isoforms of 5-α-reductase activity in intact cells by biochanin A and comparison to EGCG.
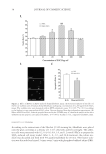
A NEW STRATEGY TO MODULATE ALOPECIA 53 repertoire of functions. Their interaction with integrin receptors at the surface of cells anchors skin cells to basement membranes and modulates cellular processes such as pro- liferation, apoptosis, differentiation, and motility (35). Laminin-511 (laminin-10) is the predominant laminin at the epithelial–mesenchymal interface of the hair follicle and is crucial for hair morphogenesis and anagen hair growth (36). Laminin-511 stimulates hair growth in vitro, and preventing its expression at the dermal papilla leads to hair regres- sion and alopecia, a defect that can be prevented by application of the purifi ed protein (37). Chemotherapy-induced alopecia has recently been associated with downregulation of laminin-511 expression, providing a new target for the prevention of this adverse effect (37). The effects of the matrix protein on hair follicle are mediated through the binding and activation of specifi c integrins at the surface of cells (38). Collagen VII. Collagen VII is a long-chain collagen synthesized and secreted by keratino- cytes and dermal fi broblasts. This matrix protein is a major component of anchoring fi brils that are specialized attachment complexes at epithelium–mesenchyme interface, in a number of tissues. In human skin, anchoring fi brils extend from the lower portion of the dermal–epidermal basement membrane to the underlying upper papillary dermis, form- ing U-like structures that entrap interstitial collagen fi bers (39). In the hair follicle, col- lagen VII is found along the BMZ outside the hair follicle and at the dermal papilla junction inside the hair bulb. Collagen VII colocalized with integrins and laminins-511 at the epithelial–mes enchymal interface, suggesting a role in hair growth (14,15). The close interaction of collagen VII with laminins and interstitial collagen provides stability to the epithelial–mesenchymal interface. Effects of acetyl tetrapeptide-3 on matrix proteins. The effect of acetyl tetrapeptide-3 (10−7 M) on the protein expression of type III collagen and total laminins in human fi broblasts (MRC5) was evaluated by selective immunofl uorescence in comparison with untreated fi broblasts. Results showed a signifi cant stimulation in the synthesis of both ECM pro- teins in the presence of the peptide (Figure 4) expression of type III collagen was in- creased by +65% while expression of laminins raised by +285% over untreated cells. Figure 4. Effects of acetyl tetrapeptide-3 (10−7 M) on the protein expression levels of type III collagen and total laminins in human fi broblasts (MRC5) (A) immunofl uorescence staining of type III collagen and lami- nins in control and acetyl tetrapeptide-3-treated cells (B) histogram representation of results. Untreated cells served as control.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)