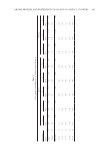
FORMULATION COMPOSITION ON CONDITIONING SHAMPOO PERFORMANCE 425 Figure 14. Effect of salt addition on micelle–polyelectrolyte interactions (14). polymers with a high molecular weight or high rigidity, such as polygalactomannans, with micelles that have high aspect ratios (occurring at higher surfactant amount) com- pared to those with low aspect ratios (occurring at lower surfactant amount). The surfac- tant micelle–cationic polymer interactions are further infl uenced by the charge density on the micelle and that of the cationic polymer. For instance, the interaction of high charge density micelles with high cationic charge density polymers such as the cationic cassia polymers in this study, which typically have higher cationic charge densities than other commonly used cationic polymers, favors high silicone and cationic deposition. Several explanations are possible. The complex may be more likely to adhere to the negatively charged hair surface. Flocculation of the silicone droplets may be more effi cient between cationic cassia polymers and highly negatively charged micelles. It is also possible that there is a different interaction (or conformation) between cationic cassia and micelles with high anionic charge compared to those with low anionic content, depending of the cat- ionic polymer charge density. The effect of ionic strength leads to a decrease in the silicone or cationic polymer deposi- tion especially for the low cationic charge density polymers such as cationic guar CG0.98 or PQ-10 1.03. This can be explained by the work done by Dubin and Oteri (15). Spe- cifi cally, these investigators showed that salt (or ionic strength) has a signifi cant effect on both coacervation and precipitation of polyelectrolytes and oppositely charged micelles due to screening of the interaction between the two components. They suggest that both forms of aggregation can be suppressed or enhanced by addition of salt (see Figure 14). Screening of electrostatic interactions between polyelectrolytes and micelles by salt means that the binding affi nity of micelles to polyelectrolytes increases with a decrease in salt concentration or decreasing ionic strength (i.e., in the initial formulation or also by shampoo dilution). As the binding affi nity increases, the system can change from one in which no micelles are bound (phase I), to a positively charged complex with few bound micelles (phase II), then to a system with suffi cient micelles for net neutrality (phase III), and fi nally to a negatively charged complex with excess bound micelles (phase IV). Similarly, the addition of salt can move the system from phase IV to phase I. Therefore, the complex [or coacervate (phase III)] can be “suppressed” or “enhanced” through changing the number
JOURNAL OF COSMETIC SCIENCE 426 of bound micelles. Thus, different coacervation and/or precipitation can be obtained from a surfactant/polymer system by changes in ionic strength or salt content. The data from this study show that the salt screening effect may infl uence the coacervation behavior and therefore the cationic and silicone deposition of the lower cationic charge density poly- mers such as cationic guar CG0.98 and PQ-10 1.03, compared to the cationic cassia polymers with higher cationic charge density. CONCLUSIONS The results show that different factors infl uence the conditioning performance of different cationic polymers. The formulation composition has a strong infl uence on the silicone and cationic polymer deposition that are primary determinants of the conditioning performance. Three parameters are highlighted to be of importance in determining silicone and cationic polymer deposition: ionic strength, average surfactant charge (micelle charge), and the total amount of surfactant. Silicone and cationic polymer deposition re- sults can be predicted to a high confi dence level using models that incorporate these three factors. There appears to be several mechanisms that are of importance in determining silicone and cationic deposition and, therefore, conditioning performance. Which of these factors is operative may depend on the cationic polymer molecular weight, cationic charge density, polymer chain fl exibility, and solubility. A summary of these factors for silicone deposition and cationic polymer deposition is in Tables III and IV, respectively. Silicone and cationic deposition data indicate that increasing the interaction between the cationic polymer and surfactant by either using a more highly charged cationic polymer, decreas- ing the aspect ratio of the surfactant structuring (lower surfactant amount), or decreasing the ionic strength (less ionic interaction shielding) may contribute to better deposition. Table III Summary of the Factors Infl uencing Silicone Deposition for Each Cationic Polymer (Increasing Factors) Silicone deposition Cationic cassia Cationic guar Cationic hydroxyethyl cellulose Surfactant amount ↓a ↓ Micelle charge ↑ ↑ ↓ Ionic strength ↓ Cationic charge a Signifi cant interaction with micelle charge (Figure 5). Table IV Summary of the Factors Infl uencing Polymer Deposition for Each Cationic Polymer (Increasing Factors) Cationic deposition Cationic cassia Cationic guar Cationic hydroxyethyl cellulose Surfactant amount ↓a ↓ ↓ Micelle charge ↑ ↑b Ionic strength ↑ ↓ Cationic charge ↑ a Signifi cant interactions with micelle charge and cationic charge (Figure 10). b Signifi cant interaction with ionic strength (Figure 12).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)