FORMULATION COMPOSITION ON CONDITIONING SHAMPOO PERFORMANCE 413 cationic charge densities of 1.9 and 3.0 mEq/g, and widely used commercially available cationic polymers such as cationic guar polymer (0.98 mEq/g) and cationic hydroxyethyl cellulose, polyquaternium-10 (PQ-10 1.03 mEq/g). Latter two polymers are chosen be- cause of their extensive use as conditioning polymers in shampoos. This study covers a wide range of polymer compositions (galactomannans and cellulosics), molecular weights, and cationic charge densities. It is known that the formulation composition has a dramatic effect on physical properties (viscosity, clarity, and turbidity) and also on the coacervation behavior (7–9). The purpose of this study is to determine the factors infl uencing the conditioning performance of shampoos, specifi cally the deposition of a small-particle-size silicone emulsion (average silicone droplet size of about 0.5 mm) and cationic polymer depositio n. EXPERIMENTAL MATERIALS Various cationic polymers such as cationic cassia derivatives of different cationic charge densities (1.9, 2.3, and 3.0 mEq/g), cationic guar (0.98 mEq/g), and cationic hydroxyethyl cellulose (1.03 mEq/g) are used in this study as illustrated in Table I. Cassia gum is a natural vegetal carbohydrate based on mannose and galactose sugars extracted from the endosperm of the seed of Cassia tora and Cassia obtusifolia. It is a member of the galactomannan family of polysaccharides with a ratio of mannose to galactose content of at least 5:1. Cassia gum can be modifi ed to generate cationic galactomannans with various levels of cationic substitution (10). The modifi cation produces cationic cassia conditioning polymers, CC1.9, CC2.3, and CC3.0, with cat- ionic charge density levels of 1.9, 2.3, and 3.0 mEq/g, respectively, available from Lubrizol Advanced Materials (Brecksville, OH). The chemical structures of the vari- ous cationic polymers used in this study are illustrated in Figure 2. Polysaccharide derivatives have a long history of use in personal care applications as thickeners, con- ditioning polymers, deposition aids, and fi lm formers. Cationic derivatives of guar gum, another galactomannan having a mannose to galactose ratio of about 2, have been successfully used in conditioning shampoos in combination with silicones to impart improved combing and sensory properties. The International Nomenclature of Cosmetic Ingredients designation for the cationic cassia polymers is cassia hydroxypropyltrimonium Table I Cationic Polymer Characterization Cationic polymer Code name Charge density (mEq/g) Molecular weight Mw (D) Cationic cassia CC1.9 1.9 600,000 Cationic cassia CC2.3 2.3 600,000 Cationic cassia CC3.0 3.0 600,000 Cationic guar CG0.98 0.98 2,000,000 Cationic hydroxyethyl cellulose PQ-10 1.03 1.03 400,000
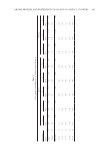
JOURNAL OF COSMETIC SCIENCE 414 chloride, for cationic guar is guar hydroxypropyltrimonium chloride, and for cationic hydroxyethyl cellulose polymer is polyquaternium-10. The molecular weight of the cat- ionic polymer can be determined by a low-angle light-scattering detector (Triple Detec- tor Array, available from Viscotek (Houston, TX), model number 302-040) coupled with two Visco-Gel C-MBHMW-3078 columns using a sample concentration of 0.6 mg/ml in a 0.05 M ammonium acetate/10% methanol solvent (at a pH of 4.0), an injection vol- ume of 100 μl, a column temperature of 30°C, and a fl ow rate of 0.9 ml/min. The surfactants used in this study are sodium lauryl ether sulfate (SLES-2), sodium lauryl sulfate (SLS), and cocamidopropyl betaine (CAPB), all commercially available Figure 2. Chemical structures of (A) cationic hydroxyethyl cellulose, (B) cationic cassia, and (C) cationic guar.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)