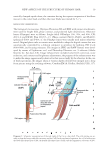
ANTIOXIDANT PROPERTIES OF FERMENTED MANGO LEAF EXTRACTS 11 Both lactobacillus and EM fermentations showed increasing tyrosinase inhibitory activi- ties with increasing concentrations of the extract. Antioxidant activity increased with increasing concentrations of the extracts. EM fermen- tations showed a higher antioxidant effect over lactobacillus fermentations through in- creased inhibition of ROS production. Cytotoxic effect also augmented with increasing concentration of fermentation extracts as well. Both fermentations demonstrated stable cytotoxicity level demonstrated as cell viability percentage 85% at different concentra- tions tested (38). The results of this study show that mango leaf extracts fermented with lactobacillus and EM impart positive effects on RAW 264.7 cells by reducing ROS pro- duction as well as enhancing cytotoxicity-related stability and antioxidant activity. One of the limitations of this study is differential time duration required to ferment each culture the difference of 7 days for EM activation versus 2 days for Lactobacillus fermentation remains a signifi cant challenge to the equivalent effi cacy assumption despite equal seed culture concentration used to prepare the MLFE and the MEFE. Longer duration required for optimal fermentation should be balanced against addi- tional antioxidant effect obtained from the use of EM. Another limitation is in the Figure 6. Inhibitory effects of the MLFE and MEFE on tyrosinase activity (%). Tyrosinase was used for the in vitro melanin synthesis. Data presented are mean ± S.D. (n = 3). Table IV Nitrite Scavenging Abilities (%) of the MLFE and the MEFE at pH 2.5 (n = 3) MLFE concentration (mg/ml) Nitrite scavenging activity (%) t p-Value MLFE MEFE 0.4 0.08 ± 0.12 0.19 ± 0.08 −1.659 0.086 2 2.21 ± 0.07 26.03 ± 0.17 −182.5 0.000 10 46.51 ± 0.06 84.93 ± 0.01 −589.0 0.000 50 94.97 ± 0.06 95.89 ± 0.03 −12.54 0.000
JOURNAL OF COSMETIC SCIENCE 12 range of cytotoxicity tests conducted. H.N. K won (38) reported the safety threshold of cell viability at 85% when comparing antioxidant effects of various mulberry root bark extracts. The mango leaf fermented extract concentrations for cell viability test- ing in this study ranged up to 2.5 mg/ml where the previous study’s safety thresholds of 85% were demonstrated. Therefore, further cell viability tests beyond the ranges tested in this study would be necessary to establish the safety threshold for mango leaf fermented extracts. In conclusion, the antioxidative effects of microbial fermentation products containing mango leaf extracts may serve as a basis for their use in developing a variety of health foods and fermentation-based beauty products. REFERENCES (1) K . E. Shin, P opular varieties of mango in Japan, K orea Inst. Sci. Technol. Information., 1, 1–7 (2012). (2) K. Katina, K. H. Liukkonen, A. Kaukovirta, H. Adlercreutz, S. M. Heinonen, and A. M. Lampi, F ermentation-induced changes in the nutritional value of germinated rye, J. Cereal Sci., 46, 348–355 (2007). (3) N. Foolad, E. A. Brezinski, E. P. Chase, and A. W. Armstrong, Effect of nutrient supplementation on atopic dermatitis in children, Arch. Dermatol., 17, E1–E6 (2012). (4) K. S. Choi, J. Y. Yang, Y. Jin, Y. M. Park, S. Kim, H. Choi, D. Lyu, and D.-S. Kim, Pest lists and their damages on mango, dragon fruit, and atemoya in Jeju, Korea, Korean J. Appl. Entomol., 52, 45–51 (2013). (5) J. S. Choi, H. Y. Kim, W. T. Seo, J. H. Lee, and K. M. Cho, Roasting enhances antioxidant effect of bitter melon (Momordica charantia L.) increasing in fl avan 3-ol and phenolic acid contents, Food Sci. Biotechnol., 21, 19–26 (2012). (6) S. M. Shin, S. Y. Mok, S. H. Lee, K. M. Cho, E. J. Cho, and H. Y. Kim, Protective effect of bitter melon (Momordica charantia) against oxidative stress, Cancer Prev. Res., 16, 86–92 (2011). (7) Y. M. Jong, S. J. Park, K. Y. Lee, J. Y. Lee, J. K. Suh, S. Y. Hwang, K. E. Park, and M. H. Kang, Anti- oxidative and antimicrobial activities of Lilium species extracts prepared from different aerial parts, Korean J. Food Sci. Technol., 39, 452–457 (2007). (8) C. H. Chui, and G. Y. M. Cheng, Growth inhibitory potential of effective micro-organism fermentation extract (EM-X) on cancer cells, Int. J. Mol. Med., 14, 925–929 (2004). (9) C. H. Chui and D. K. P. Hau, Apoptotic potential of the concentrated effective microorganism fermen- tation extract on human cancer cells, Int. J. Mol. Med., 17, 279–284 (2006). (10) M. V. Berridge, P. M. Herst, and A. S. Tan, Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol. Annu. Rev., 11, 127–152 (2005). (11) A. Lin and P. Agrawal, G lutamine decomposition in DMEM: Effect of pH and serum concentration, Biotechnol. Lett., 10, 659–698 (1988). (12) W. Horowitz, Offi cial Methods of Analysis, 13th Ed. (AOAC INTERNATIONAL, Washington DC, 1980), p.252. (13) N ational Food Research Institute (NFRI), Manuals of Quality Characteristic Analysis for Food Quality Evaluation, (National Food Research Institute, Skuba, Japan, 1990), V ol. 2, p. 61. (14) M. S. Blois, Antioxidant determination by the use of a stable free radical, Nature, 26, 1199–1200 (1958). (15) Y. H. Ahn, J. S. Yoo, and S. H. Kim, An antioxidant capacity assay using a polyvinyl alcohol-based DPPH pellet, Bull. Korean Chem. Soc., 31, 2557–2560 (2010). (16) T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods, 65, 55–63 (1983). (17) E. H, Park, S. K. Min, Y. K. Park, S. H. Park, N. H. Kim, S. J. Hwang, and S. H. Jin, S tudy for anti- viral activity of methanol extracts in indigenous plants by MTT assay, The Annual Report of Busan Met- ropolitan City Institute of Health & Environment, 21, 9–15 (2011). (18) M. B. Eslaminejad and S. Nadri, M urine mesenchymal stem cell isolated and expanded in low and high density culture system: Surface antigen expression and osteogenic culture mineralization. In Vitro Cell. Dev. Biol. Anim., 45, 451–459 (2009).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)