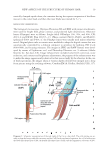
NEW ASPECTS OF THE STRUCTURE OF HUMAN HAIR 19 vertically clamped upside down, the container having the aqueous suspension of the fi bers was set to the cutter head, and then the inner blade was rotated for 5–7 s. MICROSCOPIC OBSERVATIONS The biological microscopes, Olympus Photomax LB and BHS with proper attachments, were used for bright fi eld, phase contrast, and polarized light observations. Objective lenses (Olympus) were as follows: (bright fi eld) DPlanApo 10×, 20×, and 40× CPL 20×/1.2 and LWDC Plan 40×/0.3–1.3. (Phase contrast) PL10×, PL20×, and PLL40× (polarized) P20× and P40× cf. the PlanApo lenses were usually used unless otherwise noted. Original photo tube accessories were modifi ed to adapt the digital camera that was automatically controlled by a desktop computer to optimize for lighting, ISO levels (400-6400), and focusing measures. The images in JPEG and RAW formats were devel- oped by means of Lightroom ver.3 and Photoshop Elements ver. 9 software (Adobe Systems Inc. San Jose, CA). Image enhancement included color level correction, noise reduction, and contrast and brightness adjustments the purpose of the enhancement was to make the image appear nearly identical to that seen actually by the observer. In the case of thick specimens, the images taken at various depths of fi eld were merged into a deep focus picture using the stacking software, CombineZM (A. Hadley, Sheffi eld, UK), (17). Diagram 2. Schematic representation of the side views of the Cu in a hair shaft. The cells overlap one an- other to form the honeycomb-like structure and the CuP. On the mechanical agitation, the cell is broken into the blade-like shaped part (CuB), the handle-like shaped part (CuH), and the fragmentary substance (CuB’).
JOURNAL OF COSMETIC SCIENCE 20 RESULTS AND DISCUSSION In the present study, we examined the structure of the human scalp hair shaft, utilizing a methodology that was somewhat different from the previous investigations. The hair fi - bers were subjected to either the simple swelling treatment or the enzymatic proteolysis or the S–S bond cleavage reaction, followed by random scission using rapidly rotating cutters. The hair fi bers were also physically fractured by strongly compressing the shaft with a vise. With these preparative methods, various kinds of specimens containing isolated Figure 1. The photomicrographs of the Co and Cu including the cellular parts, PLL40× objective bar 25 μm. Co, cortical cell Cu, cuticular cell CuB, the broad blade-like shaped part of Cu CuB’, the fragmen- tary substance derived from CuB CuH, the handle-like shaped part of Cu Mf, the macrofi brils of Co and Cu and N, nucleus. Hair III was warmed in an aqueous solution of papain (0.15 unit), 1 wt.% L-cysteine and 2.3 wt.% SDS at 55°C for 3 h, then subjected to the cutting process II Gentian violet staining.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)