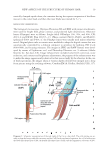
NEW ASPECTS OF THE STRUCTURE OF HUMAN HAIR 21 cells, cells’ blocks, fractured hair shaft, etc. were obtained. Like playing a jigsaw puzzle, the microphotographs of these disassembled hair parts were logically combined, resulting in the discovery of many unknown structures of the cellular components and the hair shaft itself. Some of them are schematically illustrated in Diagrams 1 and 2. The new structural features were common to all the hair samples (I–IV) examined. CORTICAL CELL The cell was easily recognized by a well-known spindle-like shape (a length of 80–90 μm and a width of 6–9 μm) as shown in Figure 1(A). The fi brous substances, which are most likely referred to as macrofi brils (Mf), were aligned in the same direction as the cell’s longitudinal axis. Though the Cos, together with a medulla, have long been considered to occupy the inner domain of a hair shaft (1–3,12) and affect the fi ber curvature (18–20), the cells were found to localize in the small space of the hair shaft, concentrically sur- rounding the medulla (M) (Figure 2). Furthermore, all the Cos appear to be grouped into more than 20 thick cord-like shaped substances [Figure 3(A)]. The substances twisted together as seen clearly in the specimens, which were prepared by physically fracturing the hair shaft [Figure 3(B) and 3(C)]. Formation of the cord-like shaped substances has not been explained well at present, but might be due to a special spatial arrangement of the Co in other words, every Co was regularly shifted a little from neighbors along the long and short axes of the cell body (Figure 4). By the way, the Co was often more deeply stained with the basic dye, Gentian violet, than the Cu the former cells were bluish purple while the latter, particularly the CuB part (vide infra), were gray or pale blue as shown in Figure 5. The facile stainability of the Co may be attributed to the high content of acidic amino acid residues of the keratin proteins involved (5,8,21). CUTICU LAR CELL On chemical treatment with an aqueous mixture of urea, SDS, and ME, the hair shaft was transformed to a hollow fi ber, gradually losing the inner substances [Figure 6(A) and Figure 2. The cross section of a hair shaft (left: no staining right: Congo red staining) bar 50 μm. The re- gion of the handle-like shaped part (CuH) of the cuticular cells (Cu) was more deeply stained by the dye than the region of the cortical cells (Co). Hair II was heated in an aqueous solution of 7 M urea, 3.5 wt.% SDS and 15 wt.% ME at 55°C for 15–30 min, followed by slicing with the cutting process III. Thereafter, about half the amount of the resulting substances was subjected to Congo red staining.
JOURNAL OF COSMETIC SCIENCE 22 6(B)] cf. the footnote.§ Amino acid analysis indicated that the hollow fi ber was approxi- mately comparable with the whole cuticles or so-called “cuticular scales” (Table I). The wall of the fi ber chiefl y comprised 7–12 overlapping scale-like substances [Figure 6(C)]. Figure 3. The cord-like shaped substances of cortical cells. (A) Hair III was treated in an aqueous solution of 8 M urea, 4 wt.% SDS and only 0.7 wt.% ME at 85°C for 2 h, then subjected to the cutting process II CBB staining PLL40× objective bar 50 μm. (B and C) The polarized light microscopic pictures of the frac- tured shaft of a hair sample III, cf. the Method 4 in the experimental section P40× objective bar 50 μm. The hair fi ber was optically anisotropic, especially in the direction of the longitudinal axis. Therefore, and the angle between the axis of the hair and the polarizer was varied properly while the angle between the polar- izer and the analyzer was set to 45° a sensitive tint plate was used. The chromatic distinction seen in the fractured hair may be chiefl y caused by the difference in birefringence or the heterogeneity in the α-crystallites of the proteins involved. §The substance, which was squeezed out from hair shafts, was almost completely dissolved in the reaction medium, amounting to 45%–70% of the starting dry weight of hair shafts. On dialysis of the mother liquor, the keratinous proteins (MW 12–60 kD) were easily obtained as an aqueous solution (21,22). The amino acid analysis of the proteins was consistent with that of the Mf of the starting natural fi bers. On oxidation treatment, the aqueous keratin solution was readily transformed to various water-insoluble biomaterials such as fi lm (21,22,23), cultivation substrata (24), microcapsules (25), and sponge (26–28).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)