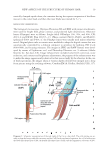
NEW ASPECTS OF THE STRUCTURE OF HUMAN HAIR 27 Figure 10. The honeycomb-like structure on the cuticular thin plate (CuP) PLL40× objective bar 50 μm cf. Figure 8. The pictures of the panels A and B were taken by focusing on the inner and outer surfaces of the plate, respectively. The gray circle in the panel A shows an example of the fusing sites of the blade-like shaped parts (CuB) of the Cu. The specimen was prepared by heating hair I in an aqueous mixture of 7 M urea, 3.5 wt.% SDS and 15 wt.% ME at 80°C for 2 h, then subjected to the cutting process III staining with Giemsa’s solution. Figure 11. (A) The medulla as a tubular substance with the wall of 1–2 μm in thickness cf. the image was enhanced using the color development software PL20× bar 30 μm. Hair III was heated in an aqueous mix- ture of 6.3 M urea, 3.4 wt.% SDS and 13 wt.% ME at 80°C for 6 h, then subjecting to the cutting process II double staining with CBB and Giemsa’s solution. (B) The medulla’s wall and the blade-like shaped parts (CuB) of the Cu bar 25 μm. It appears that the cortical cells and the handle-like shaped parts (CuH) were partially lost from the hair fi ber during the preparation of the specimen. Hair IV was warmed in an aqueous mixture of 0.3 wt.% Congo red and 0.08 wt.% SDS (without ME) at pH 5 and 65°C for 30 min, washed with water, then subjected to the cutting process III. study that the medulla was a tubular substance with a wall-like coating Figure 11 cf. Diagram 1 (position: 3-e). It was also found that the medulla, like CuB, CuP, and hollow fi ber, was stable to severe chemical treatments for instance, heating in an aqueous solu- tion of 20 wt.% ME at 50°C–80°C for 8–12 h. All of the hair samples (I–IV) showed that the medulla continuously streamed through in the center of the shaft. We have been in- vestigating the physicochemical property of the medulla and will be reported later.
JOURNAL OF COSMETIC SCIENCE 28 CONCLUSIONS The structure of the hair cells and the fi ber shaft itself is substantially revised by optical microscopic observations of the hair components. Particularly, irrespective of the hair sources, that a mature Cu has a trowel-like shape with the following distinctive features: the part with handle-like shaped part (CuH) is full of Mf and usually colored by melanin granules, while the blade-like shaped parts fuses partially to build the honeycomb-like structure and a large cuticular thin plate. The medulla is tubular, streaming through all the hair shafts examined. The physicochemical stability of hair is mainly attributed to the presence of the honeycomb-like structure, the cuticular thin plate and the Mf of the Co and Cu. The present discoveries might be useful for people, especially chemists in the hair-related fi elds, to get new insights for their studies. ACKNOWLEDGMENTS We are deeply indebted to the staff members of Department of Applied Biochemistry, Osaka University and Department of Biochemistry, Osaka Municipal Technical Research Institute for many invaluable discussions and technical assistance throughout this work. We also thank Alan Hadley for permission to use the CombineZM software. REFERENCES (1) C. R. Robbins, Chemical and Physical Behavior of Human Hair, 5th ed. (Springer-Verlag, Berlin, 2012), Chapters 1 and 2. (2) C. E. Orfanos, W. Montagna, and G. Stüttgen (Eds.), Hair Research: Status and Future Aspects (Springer- Verlag, Berlin, 1981), Part I, pp. 3–234. (3) L.N. Jones, D. E. Rivett, and D. J. Tucker, “Wool and Related Mammalian Fibers” in Handbook of Fiber Chemistry, 3rd ed., M. Lewin Ed. (CRC Press, Boca Raton, FL, 2007), pp. 331–381. (4) K. Morioka, Hair Follicle, Differentiation under the Electron Microscope (Springer-Verlag, Tokyo, 2005). (5) L. J. Wolfram and M. K. O. Lindemann, Some observations on the hair cuticle, J. Soc Cosmet Chem, 22, 839–850 (1971). (6) J. A. Swift and B. Bews, The chemistry of human hair cuticle-I: A new method for physical isolation of cuticle, J. Soc. Cosm. Chem., 25, 13–22 (1974). (7) J. A. Swift and B. Bews, The chemistry of human hair cuticle-II: The isolation and amino acid analysis of the cell membranes and A-layer, J. Soc. Cosm. Chem., 25, 355–366 (1974). (8) J. A. Swift and B. Bews, The chemistry of human hair cuticle-III: The isolation and amino acid analysis of various subfractions of cuticle obtained by pronase and trypsin digestion, J. Soc. Cosm. Chem., 27, 289–300 (1976). (9) P. Kassenbeck, “Morphology and Fine Structure of Hair,” in Hair Research Status and Future Aspects, C. E. Orfanos, W. Montagna, and G. Stüttgen. Eds. (Springer-Verlag, Berlin, 1981), pp. 52–64. (10) R. H. Rice, V. J. Wong, and K. E. Pinkerton, Ultrastructural visualization of cross-linked protein fea- tures in epidermal appendages, J. Cell Sci., 107, 1985–1992 (1994). (11) R. H. Rice, V. J. Wong, K. E. Pinkerton, and J. P. Sundberg, Cross-linked features of mouse pelage hair resistant to detergent extraction, Ana. Rec., 254, 231–237 (1999). (12) Ta. Takizawa, To. Takizawa, S. Arai, M. Osumi, and T. Sato, Ultrastructure of human scalp hair shafts as revealed by freeze-substitution fi xation, Ana. Rec., 251, 406–413 (1998). (13) R. D. B. Fraser, T. P. MacRae, and G. E. Rogers, Keratins, Their Composition, Structure and Biosynthesis (Charles Thomas, Springfi eld, 1972), p. 56–74. (14) S. Ogawa, Y. Takeda, K. Kaneyama, and T. Komoto, Electron microscopic study of permanent straight- ening hair prepared by a reduction and heat treatment process, Sen’i Gakkaishi, 64, 352–357 (2008). (15) L. N. Jones, D. J. Peet, D. M. Danks, A. P. Negri, and D. E. Rivett, Hairs from patients with maple syrup urine disease show a structural defect in the fi ber cuticle, J. Invest, Dermatol., 106, 461–464 (1996).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)