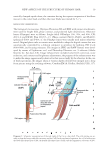
PRACTICAL SELECTING METHOD OF WAVE LOTION FOR HAIR DRESSER 43 (12). However, approximate determination of the reduction kinetics in hair was per- formed without pretreatment in order to confi rm that reduction kinetics of TGA-Na follows pseudo fi rst-order reaction and in this way compared with that of TGA-NH4 so- lutions. Both solutions were 0.6 M in thioglycolic acid and adjusted to a pH of 8.6 (see Preparation of sodium and ammonium thioglycolate solution in Experimental section). As can be seen in Figure 6(A), Wickett’s results for TGA-Na were approximately repro- duced using our simplifi ed conditions, although slight deviations were observed during the initial 6 min. In addition, it was found that the reduction proceeded much more rapidly with TGA-NH4 than with TGA-Na. Furthermore, the -ln (Ft/Fo) vs. time plot for of TGA-NH4 yielded a straight line for the initial 10 min but then deviated from a straight line thereafter. These results suggest that reduction using TGA-NH4 follows a pseudo fi rst-order reaction during the initial stage however, beyond this stage, the mech- anism of reduction is no longer the same. Notably, despite the fact that the results were Figure 6. Comparison of the reduction kinetics for the Na and NH4 salts of thioglycolic acid: Pa and –ln(Ft/Fo) vs. time. (A) Left axis: Pa/t curves for (▲) TGA–Na and ( )TGA–NH4 right axis: –ln(Ft/Fo) for ( ) TGA–Na and ()TGA–NH4 vs. time TGA–Na ( ) vs. time: y = 0.036X , r = 0.992 and TGA–NH4()vs. time: y = 0.13X, r = 0.989 only during the initial stage. Conditions salt concentration: 0.6 M pH: 8.6. Note: the plot of –ln(Ft/Fo) for TGA–NH4 vs. time also shows a linear relationship only in the initial stage of the reac- tion. (B) Optical microscopic images of the sections of white hair pretreated with NH4+ and Na salts of thioglycolic acid and stained with methylene blue. Bottom: Gray-scale image obtained by scanning along the line between the two points indicated by arrowheads on the micrographs (taken by Dr. Kawamura).
JOURNAL OF COSMETIC SCIENCE 44 obtained at a pH of 9, the results for TGA-NH4 disagreed with both Wickett’s conclu- sion (11) that TGA-Na kinetics are pseudo fi rst-order at pH 9 or below. Considering all of the above data, it can be concluded that the reduction kinetics for TGA-NH4 in hair is different than those for TGA-Na. Next, the reduction characteristics for all TGA-NH4 wave lotions listed in Table I were evaluated and found to be similar to those described above. However, the plots of -ln(Ft/ Fo) for the C–N and C–W lotions, which are very weak wave lotions, vs. time appeared to follow pseudo fi rst-order kinetics, as can be seen in Figure 7(A). To determine whether the behavior observed in these plots for the C–N and C–W lotions only occurred in the initial stages of the reactions, the reduction time was increased from 20 to 90 min. Figure 7(B) shows the Pa/t curve and plot of -ln(Ft/Fo) vs. time for the C–W wave lotion over an extended period of time (90 min). It can be clearly seen that the plot of -ln(Ft/Fo) vs. time for the fi rst 20 min in Figure 7(B) corresponds to the initial stage of the reduction, Figure 7. Characteristics of the reduction kinetics of TGA–NH4 wave lotions. (A) Left axis: Pa vs. time for ( ) C–S, (▲) C–N, and ( ) C–W Right axis: –ln(Ft/Fo) vs. time for ( ) C–S :y = 0.136X, r = 0.992 (only initial stage), ( ) C–N :y = 0.057X, r = 0.996, and ( )C–W: y = 0.36X, r = 0.996. Note: The results for C–N and C–W (weak wave lotions) suggest that these reactions were pseudo fi rst order. (B) Left axis: At an extended reduction duration of 90 min, (▲) Pa vs. time for C–W right axis: ( )–ln (Ft/Fo) for the same. Note: This reaction exhibited the same pattern as other TGA–NH4 .
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)