NEW ASPECTS OF THE STRUCTURE OF HUMAN HAIR 17 aqueous suspension of small hair fragments was mixed with a fresh 0.5 wt.% aqueous dye solution (2–3 drops) at ambient temperature for 0.5–5 h, where the dye used was Congo red, Coomassie brilliant blue G-250 (CBB), Gentian violet, 2’,7’-dibromo-4’- hydroxymercurifl uorescein sodium salt (SM), and a Giemsa’s solution (Wako Pure Chem., Osaka, Japan) (16). Sometimes the hair fragments were silver stained by soaking in aque- ous 0.2 wt.% silver nitrate, separating with a centrifuge, and exposing to bright sunlight for a while, followed by reduction with an aqueous 2 wt.% ascorbic acid at ambient temperature. After washing briefl y with water, the stained sample was placed on a micro- scope glass slide using aqueous 50 wt.% glycerol as a mounting medium and overlaid with a cover glass (Matsunami Glass No.1). A weight was placed on the cover glass (15–30 g/cm2) while sealing the glass edges with Canada balsam. Method 2: Treatment of hair fi bers with papain (a general procedure). Hair (I–IV, 0.13 g, about 1 cm in length) was incubated with occasional shaking at 55°C for 2–6 h in the pH 7/0.07 M phosphate solution (10 ml) containing papain (crude powder type from Carica papaya, about 0.15 unit), L-cysteine (0.1 g, an activator), and SDS (0.23 g). The digested fi bers were taken out with a spatula at 2-h intervals. After washing with water, the fi bers were suspended in pure water (2.5 ml) and subjected to the cutting process I or III (vide infra). The resulting hair fragments were stained and mounted to a microscope glass slide in a manner similar to that mentioned above. Method 3: Chemical treatment of hair fi bers using ME (a general procedure). Hair (I–IV, 80 mg, about 2 cm in length) was put to an aqueous mixture (10 ml) of 6.4–8 M urea, 3–4 wt.% SDS and 1–20 wt.% ME in a screw-cap glass test tube. The tube was set in a horizontal position and warmed without shaking at 50°C–85°C for 20 min–12 h. After cooling to ambient temperature, the solid remainder of the hair shaft was washed briefl y with aque- ous 0.5 wt.% ME and subjected to the cutting process II or III (vide infra). The resulting suspension of hair fragments was centrifuged at 750 g to give the precipitate that was washed with aqueous 0.2 wt.% ME, stained, and mounted on the slide glass in a manner similar to that described above. Method 4: Compression fracturing of the hair shaft. Hair (III, IV) was warmed in an aqueous solution of 7 M urea and 3.5 wt.% SDS at 55°C for 5 h. The resulting softened fi ber was washed briefl y with water, cut into about 10 mm in length, and 2 or 3 pieces were sand- wiched between a glass slide (thickness, 1.3 mm) and a cover glass (20 × 20 mm with a thickness 0.72 mm) using Canada balsam as a medium. Subsequently, the glass plates were pressed by a mini-vise at the force which was slowly increased to about 0.5–1.0 kgf, taking about 15 min. The vise with the glass plates was then stored in a refrigerator (about 10°C) in order to harden the medium, followed by taking off the vise at ambient temperature to give the specimen for microscopic observation. By this compression method, the cuticular covering of the hair shaft was usually fractured in the same direc- tion as the fi ber’s longitudinal axis. MECHANICAL CUTTING PROCESSES (I, II, AND III) The chemically and enzymatically pretreated hair fi bers (obtained in the aforementioned methods 1–3) were randomly chopped using the following three kinds of cutters. (i) A stainless steel gear (18 teeth, 8 mm diameter and 4 mm height) was placed in the aqueous suspension of the pretreated hair fi bers at ambient temperature and rotated by a
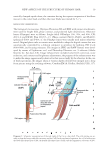
JOURNAL OF COSMETIC SCIENCE 18 small electric motor in the speed of 3500–4000 rpm for 3–10 s. This cutting method was found to effectively separate the blade-shaped parts (so-called “scale”) from Cu. (ii) A soft polyethylene gear having four teeth (each, 4 mm length × 2 mm width × 0.5 mm thickness) was rotated in an aqueous suspension of the pretreated hair fi bers in water (3–5 fi ber/ml) at the speed of about 500 rpm and ambient temperature for about 10 sec. This cutting process was used for the chemically or enzymatically pretreated hair fi bers to coarsely chop the shaft. (iii) A nose hair cutter (Hitachi, model BM-03, Tokyo, Japan) was used to obtain the very small hair fragments including the cells and cellular blocks. The outer steel blade with 9 slits (each slit: 0.8 mm width and 3.5 mm height) was designed to guide hair into a pair of inner blades (9 mm edge length, 2 mm depth, and 0.1 mm thickness). The pretreated hair fi bers in distilled water (1.5–2.0 cm3) were shortened with scissors to 1–5 mm length and put into the transparent plastic parabolic container (22 mm base diameter and 32 mm height), which was originally designed as the cap of the cutter head. The cutter body was Diagram 1. Schematic representation of the structures of a human hair shaft, the cortical cell (Co) and the cuticular cell (Cu). Co takes a spindle-like shape. The cells gather together to form more than 20 thick cord- like shaped substances. Cu is a trowel-like shaped substance, consisting of a handle-like-shaped part (CuH) and a CuB. CuH is similar in dimensions to Co, and both are fi lled with plenty of macrofi brils (Mf). Cu overlap one another and fuse partially, displaying tile roof-like and honeycomb-like patterns in the outer and inner surfaces of the CuB region, respectively. CuB, in its basal area, merges completely with other units to produce the CuP, which encircles the inner cellular components. The nucleus (N) of Cu is in CuB. Medulla (M) is a tubular substance in the center of the hair fi ber. The size (micrometer in unit) of the cells and the cellular components was measured by the use of an optical microscope and not corrected with the swelling degree of a hair shaft.‡
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)