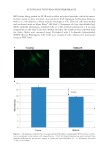
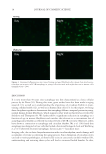
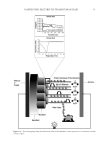
J. Cosmet. Sci., 67, 1–11 (January/February 2016) 1 Investigation of hair dye deposition, hair color loss, and hair damage during multiple oxidative dyeing and shampooing cycles GUOJIN ZHANG, ROGER L. MCMULLEN, and LIDIA KULCSAR, Ashland, Inc., Bridgewater, NJ 07004. Accepted for publication October 5, 2015. Synopsis Color fastness is a major concern for consumers and manufacturers of oxidative hair dye products. Hair dye loss results from multiple wash cycles in which the hair dye is dissolved by water and leaches from the hair shaft. In this study, we carried out a series of measurements to help us better understand the kinetics of the leaching process and pathways associated with its escape from the fi ber. Hair dye leaching kinetics was measured by suspending hair in a dissolution apparatus and monitoring the dye concentration in solution (leached dye) with an ultraviolet–visible spectrophotometer. The physical state of dye deposited in hair fi bers was evaluated by a refl ectance light microscopy technique, based on image stacking, allowing enhanced depth of fi eld imaging. The dye distribution within the fi ber was monitored by infrared spectroscopic imaging of hair fi ber cross sections. Damage to the ultrafi ne structure of the hair cuticle (surface, endocuticle, and cell membrane complex) and cortex (cell membrane complex) was determined in hair cross sections and on the hair fi ber surface with atomic force microscopy. Using differential scanning calorimetry, we investigated how consecutive coloring and leaching processes affect the internal proteins of hair. Further, to probe the surface properties of hair we utilized contact angle measurements. This study was conducted on both pigmented and nonpigmented hair to gain insight into the infl uence of melanin on the hair dye deposition and leaching processes. Both types of hair were colored utilizing a commercial oxidative hair dye product based on pyrazole chemistry. INTRODUCTION Hair dyeing is a common cosmetic practice in the beauty industry and is carried out in the salon by professional cosmetologists as well as at home by the consumer. It is an especially common procedure for individuals with gray hair, but is also practiced by members of the younger population. Usually, hair is periodically dyed (every 4–6 weeks) due to new hair growth. The most suitable kinds of coloration come from the use of oxidative (or perma- nent) dyes, which offer a great variety of shades and increased wash fastness. In general, oxidative dyes consist of primary intermediates, color couplers, and oxidizing agents (1,2). During the coloring process, the primary intermediates are activated by hydrogen peroxide and then react with color couplers inside the hair shaft. The newly formed molecules inside Address all correspondence to Roger McMullen at rmcmullen@ashland.com.
JOURNAL OF COSMETIC SCIENCE 2 the hair are colored and large enough so that they do not easily leach out from the hair structure. They also have affi nity to hair, presumably due to van der Waals or other nonco- valent interactions between the dye and the internal structural components of the hair, al- lowing them to remain in the hair structure even during shampooing or rinse out (3). Hair dye deposition and water fastness is infl uenced by the permeability of primary inter- mediates into the hair and the health condition of the ultrafi ne structural components of hair. Typically, oxidative hair dyeing is carried out at high pH (~10), utilizing ammonia or ethanolamine, causing cuticle swelling and facilitating entry of the dye intermediates into the fi ber structure as well as to decompose hydrogen peroxide so that the dyes can be activated (4). This high pH damages many of the lipids on the surface of the hair, making it a more hydrophilic substrate with greater capacity to absorb ingredients (5). In addi- tion, bleaching, dyeing, and other harsh chemical treatments damage the ultrafi ne struc- ture of hair leading to an overall more porous, or open structure, allowing dye molecules to easily diffuse into and out of the hair (6–8). The ease with which a molecule diffuses into or out of the fi ber is dependent on a number of factors including its molecular size (9). In fact, kinetic studies of dye removal by Wong et al. demonstrated that smaller dyes rinse out much more readily than larger dyes (10). In addition to surface damage, oxidizing agents in permanent hair dye systems also dis- solve melanin and oxidize hair keratin substrate (8). Therefore, both the hair surface bar- rier of the cuticle and the ultrafi ne structure of the cortex are greatly changed. To date, there has not been a comprehensive study on the mechanism of hair dye deposition and leaching pathways published in the literature due to the complexity of hair damage caused by the dyeing process. In this study, we investigated hair damage by consecutive dyeing– shampooing equivalent cycles and elucidated dye deposition and leaching pathways. This information will be helpful for scientists to develop improved technologies that minimize the amount of damage in the coloring process, prevent color fading from washout, and even create specialized products that restore health and brilliance to colored hair. MATERIALS AND METHODS A number of experimental procedures and instruments were used to gain a better under- standing of the dye leaching process and the damage associated with the bleaching and dyeing of pigmented and nonpigmented hair. As already mentioned above, instrumental techniques were employed to investigate the morphological characteristics of the ultrafi ne structure of hair, and consisted of atomic force microscopy (AFM), differential scanning calorimetry (DSC), and dynamic contact angle analysis. In addition, we monitored the deposition of dye within the fi ber structure using refl ected light microscopy and Fourier transform infrared spectroscopy (FT-IR) spectroscopic imaging. Further, studies of the kinetics of dye leaching were carried out by exposing dyed hair to a number of rinse cycles with water while monitoring the aqueous dye concentration. HAIR TRESS PREPARATION The experiments were conducted on both European white and dark brown hair purchased from International Hair Importers (Glendale, NY). Hair tresses were prepared by gluing ~2 g of fi bers together at the hair root to a Plexiglas tab with Duco cement. The resulting dimensions of the hair tresses were 6.0 inches in length by 1.25 inches in width. Hair
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)