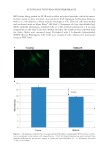
HAIR DAMAGE DURING MULTIPLE OXIDATIVE DYEING AND SHAMPOOING 7 with the aim to “close” the cuticle and, of course, prevent the leaching of dyes from the interior of the fi ber. Shampooing plays a major role in the color fastness of hair. In the experiments conducted in this study only the infl uence of water on hair dye leaching is considered. It is very likely that results may be different in presence of surfactants. Dye leaching from dark brown hair is generally faster than from white hair. This trend is more obvious in the later stages of dye leaching kinetics, which refl ects that dye mole- cules are transported from the cortex region through the cuticle, and then to the solution. More than likely, more pores and channels are created in the dark brown hair during the dyeing process when melanin granules are dissolved. This would provide extra pathways for dye molecules to leach out. These results are in agreement with previous studies that demonstrated that hair color fading is greater in pigmented than nonpigmented hair when exposed to solar radiation in combination with shampooing cycles (14). INVESTIGATION OF HAIR DAMAGE AND HAIR DYE LOSS DURING MULTIPLE OXIDATIVE DYEING–LEACHING CYCLES The hair surface becomes more hydrophilic during the dyeing process. The dyeing proce- dure removes most lipids from the hair surface since it is generally performed under alka- line conditions. After the fi rst cycle of the dyeing–leaching process, the contact angle of hair decreases from 104° for virgin hair to 84° for dyed-leached hair. There is no visible damage in the internal structure from AFM observations after the fi rst dyeing–leaching cycle alone. However, if a prebleach process is conducted prior to the hair dyeing process, Figure 3. A photomicrograph of the freshly dyed hair surface of dark brown hair obtained by refl ectance light microscopy (scale bar: 10 microns).
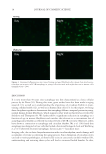
JOURNAL OF COSMETIC SCIENCE 8 AFM (Figure 4) reveals that the fi ber’s internal structure is severely damaged. Cracks (over 100 nm in diameter) and holes are observed in the cuticle (e.g., cell membrane complex, endocuticle) and cortex (e.g., cell membrane complex). Such a result suggests that the damage caused by the bleaching process facilitates dye loss from the internal structure of the hair. To evaluate hair protein damage during the consecutive dyeing–leaching cycles, protein Td of both dyed European white and dark brown hair were measured after each dyeing and leaching cycle. The results are displayed in Figure 5. The fi rst three column bars in each chart demonstrate that Td decreases with consecutive dyeing–leaching cycles, indi- cating that consecutive dyeing cycles progressively degrade hair proteins. More specifi - cally, the amorphous matrix becomes more viscous (or plastic) due to damage to its proteins. It should be noted that Td represents the denaturation temperature of crystalline phase of the hair however, changes to the amorphous matrix (which provides support for the crystalline phase) infl uence the value of Td. Plasticization with water, e.g., shifts Td to lower temperature, while cross-linking hair (making the amorphous matrix more brittle) with reactive ingredients increases Td. The last two column bars in each chart in Figure 5 refl ect how the leaching process alone affects the hair protein structure. We found that hair dyeing alone affects Td more than hair dyeing in combination with leaching. Perhaps the leaching process aids in the recov- ery of hair protein structure. Dyeing the hair results in a large increase in pH to ~9. It requires extensive rinsing to lower the pH to 6.5. It is possible that when conducting DSC measurements at high pH the hair protein is in a highly compromised state. These fi ndings are consistent in both pigmented and nonpigmented hair. Figure 4. AFM images acquired from hair cross sections in (A) virgin hair and (B) bleached then dyed hair. The images in (A) and (B) on the left side are of both cuticle and cortex the images in (A) and (B) in the middle are from the cuticle region and the images in (A) and (B) on the right side are from the cortex region.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)