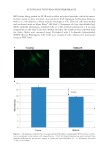
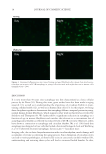
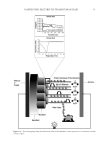
HAIR DAMAGE DURING MULTIPLE OXIDATIVE DYEING AND SHAMPOOING 3 tresses were precleaned with a 3% ammonium lauryl sulfate solution, rinsed thoroughly, and dried prior to use in the experiments. Hair was then subjected to a bleaching regimen for 30 min with Clairol BW2 (Procter & Gamble, Cincinnati, OH) beaching powder and 20-volume hydrogen peroxide (Clairoxide 20 Procter & Gamble). MULTIPLE HAIR DYEING–LEACHING CYCLES In this study, hair was subjected to a regimen that consisted of a dyeing step followed by a dye leaching step, which was carried out by immersing freshly dyed hair fi bers in water for 30 min at 40°C (called one cycle). Five dyeing–leaching cycles were conducted on both white and dark brown hair. During the dyeing steps, 12 hair tresses were dyed with Textures and Tones 4R (Red Hot Red) hair dye (Procter & Gamble, Cincinnati, OH) for 40 min. Hair tresses were then rinsed for 2 min under hot water (~38°C) and excess water was removed by forming a squeegee with the index and middle fi ngers and running them along the length of the tress. Hair tresses were then dried with a hair blow dryer (tem- perature set to medium). During the dye leaching process, each freshly dyed hair tress was suspended in a vessel containing 500 ml of water at 40°C with continuous stirring at 50 rpm. The total dye leaching time is 30 min. Leached dye was measured with an auto- mated dissolution system, consisting of a VK7000 dissolution testing apparatus (Varian Inc., Cary, NC) and an ultraviolet–visible spectrophotometer equipped with seven fl ow cells and a fl ow pump (Agilent Technologies, Santa Clara, CA). The dye concentration in solution was determined by measuring the absorbance at 490 nm. T he amount of the dye leaching from hair was calculated from the ratio of absorbance to the weight of the hair tress. The continuous dye release from hair fi bers within 30 min was measured at differ- ent times with a 2-min interval. Six repetitions per sample were measured. Hair dye leaching cycles were performed by rinsing with water. Surfactant was not added as it in- teracts with hair dye and interferes with dye detection. FT-IR SPECTROSCOPIC IMAGING A 1-cm-long hair bundle was cut from the middle of the hair tresses and mounted on the top of a sample holder by embedding in ice. The hair bundle was then microtomed at -30°C into 5-μm-thick cross sections with a Leica CM 1850 cryostat (Leica Microsystems Inc., Bannockburn, IL). Hair cross sections were collected onto CaF2 windows for IR imaging. This preparation technique avoids any possibility of contamination with embedding or fi xing medium. Hair cross sections were imaged with a PerkinElmer Spotlight system (PerkinElmer, Inc., Waltham, MA) that couples a FT-IR spectrometer with an optical microscope. The system consists of a linear array mercury cadmium telluride detector and an automated high precision x-y sample stage. Images were acquired with a 6.25 μm step size, eight scans for each spectrum, and 8 cm-1 spectral resolution. IR spectra were analyzed using ISys 5.0 software (Malvern Instruments, Inc., Malvern, Worcestershire, UK). ATOMIC FORCE MICROSCOPY The hair cross sections were prepared by the following procedure. A single hair fi ber was hung in the center of a cylinder. Buehlers Epoxicure™ Resin (Buehler, Lake Bluff, IL) and Buehlers Epoxicure™ Hardener (Buehler) were mixed (weight ratio of 5:1) and slowly poured into a cylinder. To ensure the hair fi ber remained vertically positioned in epoxy, one end of the hair fi ber was attached to a thin pole, and the other end was tied with an
JOURNAL OF COSMETIC SCIENCE 4 appropriate weight. The epoxy cures slowly at room temperature. After 24 h of curing, the epoxy containing the embedded hair fi ber was taken out of the cylinder and cut into ~2 mm thick sections perpendicular to the longitudinal axis of the hair fi ber. A standard metallography polishing technique was to use to polish the epoxy until a clean cross- sectional interface of hair was obtained. AFM images of the hair cross section were ac- quired using a Multimode Nanoscope IIId supplied by Bruker Corporation (Billerica, MA) at ambient conditions (22°C, 50% humidity) in the contact mode. A sharp nitride lever probe combining a sharp silicon tip with a silicon nitride cantilever was used for the topographic imaging acquisition. The nominal radius of the tip was about 2 nm and the spring constant of the cantilever is 0.06 N/m. A scan rate of 2 Hz was used for all mea- surements. The data collection was set to both the height and defl ection channels. OPTICAL MICROSCOPY Images of the hair surface were collected in the refl ected light microscopy mode using an Olympus BX50 (Olympus America, Center Valley, PA) optical microscope equipped with 10× (UMPlanFL 10×/0.30), 20× (LMPlanFL 20×/0.40), 50× (LMPlanFL 50×/0.50), and 100× (LMPlanFLN 100×/0.80) objectives. Equipped with a 10× eyepiece and an addi- tional 2× objective, magnifi cations of roughly 200×, 400×, 1000×, and 2000× were obtained in the fi nal images. The microscope also contains a motorized z-stage allowing z-stacks to be generated. Each fi nal image was obtained by generating an image stack and then using an algorithm to combine in focus details in each image of the stack into one fi nal image. DYNAMIC CONTACT ANGLE ANALYSIS An Attension tensiometer was used to determine contact angle (Biolin Scientifi c, Stockholm, Sweden). A single hair fi ber, cut to approximately 1 cm near the root, was immersed approximately 3 mm into deionized water and the advancing contact angle was mea- sured. The required hair diameters were measured using a handheld micrometer. Ten fi bers in each hair tress were measured. DIFFERENTIAL SCANNING CALORIMETRY A Q2000 differential scanning calorimeter (TA Instruments, New Castle, DE) was employed using pressure resistant, high volume stainless steel pans. Samples consisted of 8–12 mg of cut (2–5 mm) hair fi bers, along with roughly 55 (±1.5) mg of deionized water. Each pan was sealed and allowed to sit for at least 6 h at room temperature to ensure equilib- rium water content and distribution within the hair fi bers. Heating at a rate of 2°C/min was performed from 22° to 190°C in standard mode. Three repetitions per lot were con- ducted. TA Universal Analysis 2000 (TA Instruments, New Castle, DE) was used in conjunction with Windows 4.7A (Microsoft Corporation, Redmond, WA) to determine the denaturation temperature (Td) and enthalpy of denaturation (ΔH). RESULTS AND DISCUSSION To gain more insight into the dye leaching process, we utilized a dissolution apparatus and monitored the amount of dye leached from hair as a function of rinse time. We studied both pigmented and nonpigmented hair to understand the contribution of melanin dis- solution to the dye leaching process. Experiments were carried out using a hair dye based
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)