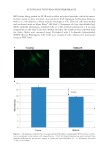
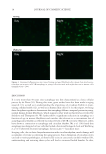
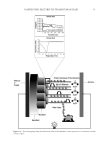
HARVESTING ELECTRICITY FROM HUMAN HAIR 35 (9) J. E. Mc Ginness, Mobility gaps: a mechanism for band gaps in melanins. Science, 177, 896–897 (1972). (10) J. McGinness, P. Corry, and P. Proctor,, Amorphous semiconductor switching in melanins. Science, 183, 853–855 (1974). (11) M. Abbas, F. D’Amico, L. Morresi, N. Pinto, M. Ficcadenti, R. Natali, L. Ottaviano, M. Passacantando, M. Cuccioloni, and M. Angeletti, Structural, electrical, electronic and optical properties of melanin fi lms. Eur. Phys. J. E: Soft Matter. Biol. Phys., 28, 285–291 (2009). (12) A. B. Mostert, B. J. Powell, F. L. Pratt, G. R. Hanson, T. Sarna, I. R. Gentle, and P. Meredith, Role of semiconductivity and ion transport in the electrical conduction of melanin. Proc. Natl. Acad. Sci. USA, 109, 8943–8947 (2012). (13) M. Marsh, and K. Earp, The electrical resistance of wool fi bres. Trans. Faraday Soc, 29, 173–192(1933). (14) S. Baxter, Electrical conduction of textiles. Trans. Faraday Soc., 39, 207–214 (1943). (15) J. Algie, and J. Downes, and B. Mackay, Electrical conduction in keratin. Text. Res. J., 30, 432–434 (_1960). (16) G. King, and J. Medley, Effect of polar vapours on the direct-current conductance of keratin and nylon. Nature, 160, 438-438 (19 47). (17) G. King, and J.Medley II, The infl uence of temperature and adsorbed salts on the DC conductivity of polar polymer adsorbate systems. J. Colloid Sci., 4, 9–18 (1949). (18) G. King, and J. Medley, DC conduction in swollen polar polymers. I. electrolysis of the keratin-water system. J. Colloid Sci., 4, 1–7 (1949). (19) M. Feugh elman, The sorption of water by dry keratin fi bers in atmospheres above 90% RH. J. Appl. Polym. Sci., 2, 189–191 (1959). (20) M. Feughelman, A two-phase structure for keratin fi bers. Text. Res. J, 29, 223–228 (1959). (21) N. E. Dorsey, Properties of ordinary water-substance. Chem. Eng. News, 18, 215 (1940). (22) N. Bjerrum, Structure and Properties of Ice, Dan. Mat. Fys. Medd, 27, 1 (1951). (23) I. Oshida, Y. Ooshika, and R. Miyasaka, Proton Transfer in Hydrogen Bond and Its Participation in π-Electron Systems. J. Phys. Soc. Jpn, 10, 849–859 (1955). ( 24) P. Ball, Water as an active constituent in cell biology. Chem. Rev., 108, 74–108 (2008). (25) D. Porter, and F. Vollrath, Water mediated proton hopping empowers proteins. Soft Matter, 9, 643–646 (2013). (26) B. Tulachan, S. K. Meena, R. K. Rai, C. Mallick, T. S. Kusurkar, A.K. Teotia, N. K. Sethy, K. Bhargava, S. Bhattacharya, A Kumar, R. K Sharma, N. Sinha, S. K Singh, and M. Das Electricity from the Silk Cocoon Membrane. Sci. Rep., 4, 5434 (2014). (27) A. O. Evans, J.M. Marsh, and R. R. Wickett, The uptake of water hardness metals by human hair. J. Cosmet. Sci., 62, 383–391(2011) (28) R. F. Schwenker, and J. H Dusenbury, Differential thermal analysis of protein fi bers. Text. Res. J., 30, 800–801 (1960). (29) W. Humphries, D. Miller, and R. Wildnauer, The thermomechanical analysis of natural and chemically modifi ed human hair. J Soc Cosmet Chem., 23, 359–370 (1972). (30) P. Milczarek, M. Zielinski, and M. Garcia, The mechanism and stability of thermal transitions in hair keratin. Colloid Polym. Sci., 270, 1106–1115 (1992). (31) C. J. T. de Grotthuss, ‘‘Sur la de´composition de l’eau et des corps qu’elle tient en dissolution a` l’aide de l’e´lectricite´ galvanique’’. Ann. Chim., 58, 54–73 (1806). (32) E. Hückel, Theorie der Beweglichkeiten des Wasserstoff- und Hydroxylions in wässriger Lösung. Z. Elektrochem., 34, 546–562 (1928). (33) A. E. Stearn, and Eyring, J. The deduction of reaction mechanisms from the theory of absolute rates. J. Chem. Phys., 5, 113–124 (1937). (34) M. L. Huggins, Hydrogen bridges in ice and liquid water. J. Phys. Chem, 40, 723–731 (1936). (35) J. D. Bernal, and R. H. Fowler, R. H. Atheory of water and ionic solution, with particular reference to hydrogen and hydroxyl ions. J. Chem. Phys., 1, 515–548 (1933). (36) G. Wannier, Die Beweglichkeit des Wasserstoff- und Hydroxylions in wässeriger Lösung. Ann. Phys., (Leipz.) 24, 545–590 (1935). (37) W. Doster, The dynamical transition of proteins, concepts and misconceptions. Eur. Biophys. J., 37, 591–602 (2008). (38) J. Guan, D. Porter, and F. Vollrath, Thermally Induced Changes in Dynamic Mechanical Properties of Native Silks. Biomacromolecules, 14, 930–937 (2013). (39) K. Tian, D. Porter, J Yao, Z Shao, and X. Chen, Kinetics of thermally-induced conformational transi- tions in soybean protein fi lms. Polymer, S1 11, 2410–2416 (2010).
JOURNAL OF COSMETIC SCIENCE 36 (40) I. Oshida, Y. Ooshika, and R. Miyasaka, Proton transfer in hydrogen bond and its participation in p-electron systems. J. Phys. Soc. Jpn, 10, 849–859 (1955). (41) E. Schauenstein, E. Treiber, W. Berndt, W. Felbinger, and H. Zima, Ultraviolett-absorptions spektren von seidenfi broin und cellulose in lithiumbromidlo¨sung. Monatsh, 85, 120–139 (1954). (42) D. Porter, and F. Vollrath, Water mediated proton hopping empowers proteins. Soft Matter, 9, 643–646 (2013). (43) P. Ball, Water as an active constituent in cell biology. Chem. Rev., 108, 74–108 (2008). (44) D. Porter, and F. Vollrath, Water mobility, denaturation and the glass transition in proteins. Biochim. Biophys. Acta, 1824, 785–791 (2012). (45) L. Pauling, The nature of the chemical bond and the structure of molecules and crystals: an introduction to modern structural chemistry. (Cornell University Press, Ithaca, 1960), Vol 18. (46) D. Porter, and F. Vollrath, The role of kinetics of water and amide bonding in protein stability. Soft Matter, 4, 328–336 (2008). (47) W. W. Cleland, and M. M Kreevoy, Low-barrier hydrogen bonds and enzymic catalysis. Science, 264, 1887–1890 (1994). (48) M. E. Tuckerman, D. Marx, M. L Klein, and M. Parrinello, On the quantum nature of the shared proton in hydrogen bonds. Science, 275, 817–820 (1997). (49) D. Marx, M. E Tuckerman, J. Hutter, M. Parrinello, The nature of the hydrated excess proton in water. Nature, 397, 601–604 (1999).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)