J. Cosmet. Sci., 67, 21–36 (January/February 2016) 21 Harvesting electricity from human hair BRINDAN TULACHAN, SUSHIL K. SINGH, DEEPU PHILIP, and MAINAK DAS, Biological Sciences & Bioengineering, Indian Institute of Technology Kanpur, Kanpur 208016, UP, India (B.T.) Solid State Physics Laboratory, DRDO, Delhi 110054, India (S.K.S.) Industrial & Management Engineering, Indian Institute of Technology Kanpur, Kanpur 208016, UP, India (D.P.) and Design Program, Indian Institute of Technology Kanpur, Kanpur 208016, UP, India (D.P., M.D.). Accepted for publication November 3, 2105. Synopsis Electrical conductivity of human hair is a debatable issue among hair experts and scientists. There are unsub- stantiated claims that hair conducts electricity. However, hair experts provided ample evidence that hair is an insulator. Although wet hair exhibited drastic reduction in resistivity scientists regarded hair as a proton semiconductor at the best. Here, we demonstrate that hair fi laments generate electricity on absorbing water vapor between 50° and 80°C. This electricity can operate low power electronic systems. Essentially, we are exposing the hydrated hair polymer to a high temperature (50°–80°C). It has long been speculated that when certain biopolymers are simultaneously hydrated and exposed to high temperature, they exhibit signifi cant proton hopping at a specifi c temperature regime. This happens due to rapid movement of water molecules on the polymer surface. This lead us to speculate that the observed fl ow of current is partly ionic and partly due to “proton hopping” in the hydrated nano spaces of hair fi lament. Such proton hopping is exceptionally high when the hydrated hair polymer is exposed to a temperature between 50° and 80°C. Differential scanning calorimetry data further corroborated the results and indicated that indeed at this temperature range, there is an enormous movement of water molecules on the hair polymer surface. This enormously rapid movement of water molecules lead to the “making and breaking” of innumerable hydrogen bonds and thus resulting in hopping of the protons. What is challenging is “how to tap these hopping protons to obtain useful electric- ity?” We achieved this by placing a bundle of hair between two different electrodes having different electro negativities, and exposing it to water vapor (water + heat). The two different electrodes offered directionality to the hopping protons and the existing ions and thus resulting in the generation of useful current. Further, by continuously hydrating the polymer with water vapor, we prolonged the process. If this interesting aspect of polymer is exploited further and fi ne tuned, then it will open new avenues for development of sophisticated polymer-based systems, which could be used to harvest electricity from waste heat. INTRODUCTION Human hair is a hot topic of debate on its electrical conductivity properties (1–5). To better understand the electrical properties of hair, the architecture of human hair is to be carefully examined (6–8). Human hair is a self-assembly of concentric, cylindrical, and structural composites with each concentric cylinder having different compositions and associated functional properties. It resembles monofi laments under microscope, Address all correspondence to Mainak Das at mainakd@iitk.ac.in
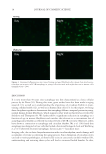
JOURNAL OF COSMETIC SCIENCE 22 with roof tiles like overlapping scales, till the tip of the fi ber. The outermost concentric cylinder called cuticle consists of plate-shaped cells, scales, which overlap both longitudinally and peripherally. Cuticle is mainly composed of structural and free lip- ids surrounding the dead cells with inner volume of the cells fi lled with keratin pro- tein. The middle layer is the bulk of the volume consisting of spindle-shaped dead cells fi lled with α-keratin and keratin-associated proteins. However, the innermost cylindri- cal yet discontinuous layer consists of cells fi lled with melanin pigments, which is known for its semiconductor properties (9–12) and ultraviolet absorption capabilities (Figure 1 A, B). The overall architecture of the hair resembles a GAU-8 Avenger gun barrel of late Nineteen seventies (1970s) in nanoscale, where the metallic barrel is replaced with “protein nano- tubes”. Each spindle-shaped dead cell in cortex consists of fi ve to eight macrofi brils. Individual macrofi bril comprises of 500–800 microfi brils, where each microfi bril is made up of seven to eight protofi laments. Each protofi lament is a self-assembly of four chain structures called tetramer of α-keratin and keratin-associated proteins (Figure 1 C). α-Keratin protein is the major component of human hair (including wool), horns, nails, hooves, and claws of mammals (5,7,8). Eminent hair experts have provided ample proof that α-keratin protein in hair is an insulator (4,5,13–15) It has been demonstrated that resistivity value of hair changes with water content. The wool–water system with 7% water content, exhibited a resistivity of 3 × 1012 ohm cm at room temperature whereas, at 25% of water con- tent, the resistivity decreased to 6 × 106 ohm cm at room temperature. It has been concluded that even at high moisture content, α-keratin is a poor conductor of electricity (4,5,13–20). The scientists considered hair as a proton semiconductor to explain the limited electrical conductivity of a keratin–water system, which is the same as that of ice, nylon–water, and cellulose–water system (4,5,14,21–26). This conduction mechanism depends on a con- tinuous hydrogen bond network that is formed between the water and keratin molecules. This network facilitates conduction by proton hopping, which explains why wet hair exhibited lower resistivity. Figure 1. Anatomy of hair. (A) Longitudinal section of a single hair fi ber. (B) Longitudinal section of the hair showing the detailed cellular architecture. Transverse cross-section showing the large cortical region. (C) Arrangement of α-keratin protein inside the cortical cells.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)