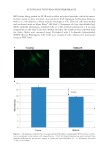
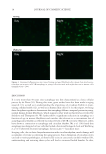
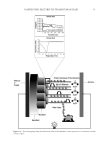
HARVESTING ELECTRICITY FROM HUMAN HAIR 33 Figure 14. Proton hopping along the α-keratin tubes of hair fi lament, when exposed to a continuous stream of water vapor.
JOURNAL OF COSMETIC SCIENCE 34 fact that, the similar electrodes could not offer suffi cient potential difference, which would result in giving directionality to the fl ow of ionic charge carriers. The fundamental question which is needed to be discussed is the generation and mobility of large number of protons on the hydrated biopolymer surface between 50° and 80°C. It was Grotthuss’s study of “structural diffusion”, carried out nearly two centuries ago, which offered an explanation to such anomalous high mobility of protons, in “‘liquid water-polymer interface” (31–49). Subsequent refi nement of this concept leads to the idea of thermal hopping (32,33), solvation effects (34), and proton tunneling (35,36). In the light of this, we have attempted to answer the key question of this study “what is the effect of water vapor on hair that resulted in the drastic increase in its conductivity?” We speculate that apart from the inherent ions which are present in the hair, at least three other events are occurring in tandem in the presence of water vapor (Figure 14). First, the protein fi lament is continuously hydrated by water vapor and water molecules occupy the vacant nano spaces of keratin tubes. These water molecules form an extensive hydrogen-bonding network along the length and breadth of the protein nanotubes. Second, this hydrogen-bonding network is perturbed by the thermal energy contained in water vapor forcing continuous rearrangement of the hydrogen bond network. This rear- rangement generates large number of hopping protons, which almost forms a continuous proton wire along the protein nanotubes (23–26). Third, thermal studies of keratin–water system demonstrated the rapid movement of water molecules over the hair protein surface at temperatures between 50° and 155°C (28–30). This suggests that the proton formation and proton hopping rate is further en- hanced by water vapor and the continuous proton wire ensures rapid transport of protons ensuring the fl ow of current (31–49). Earlier we observed similar increase in electrical conductivity in the silk–water vapor and cellulose–water vapor systems (26). Hence, from this study, a simple bioelectric device using human hair to harness measureable electricity was developed. Although we set out to revisit a long-debated controversial ques- tion of cosmetic industry, we are penning the epilogue for a waste heat management system for thermal and nuclear power plants where saturated water vapor is a waste byproduct. Thus, hair research, which remained confi ned to the cosmetics sciences, could make its way to the burgeoning arena of green and sustainable energy in future. REFERENCES (1) E. M. Fozard, Method and apparatus for removal of superfl uous hair. Patents US2888927 A: 1959. (2) R. F. Wagner Jr., J. M. Tornich, and D. J Grande, Electrolysis and thermolysis for permanent hair re- moval. J. Am. Acad. Dermatol., 12, 441–449 (1985). (3) http://www.hairfacts.com/hair-removal-methods/doubtful-hair-removal-methods/electric-tweezers- warning/ (4) M. Feughelman, The physical properties of alpha keratin fi bers. J. Soc. Cosmet. Chem. , 33, 385–406 (1982). (5) M. Feughelman, Mechanical properties and structure of alpha-keratin fi bres: wool, human hair and related fi bres. (UNSW Press, Sydney, Australia, 1997), 5, 5. (6) J. Bradbury, The structure and chemistry of keratin fi bers. Adv. Protein Chem., 27, 111–211 (1973). (7) C. Popescu, and H. Höcker, Hair—the most sophisticated biological composite material. Chem. Soc. Rev., 36, 1282–1291 (2007). (8) C. Popescu, and H. Höcker, Cytomechanics of hair: basics of the mechanical stability. Int. Rev. Cell Mol. Biol., 277, 137–156 (2009).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)