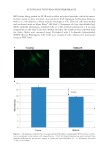
HAIR DAMAGE DURING MULTIPLE OXIDATIVE DYEING AND SHAMPOOING 5 on pyrazole chemistry, which is often employed in hair dye products designed to provide red shades. Of the dyes currently used in the market place, the pyrazole dye in this study provides one of the most intense shades of red, and is especially prone to shampoo or water removal. Therefore, this dye chemistry is a good choice to better understand the factors that control color loss. Several techniques were employed to provide measurements of the physicochemical state of the hair, focusing on its surface properties (dynamic contact angle analysis) and ultra- fi ne structure (AFM and refl ected light microscopy). Overall, we fi nd signifi cant damage in both the surface and internal components of hair due to bleaching and dyeing. Further- more, dye penetration profi les into the fi ber were monitored with FT-IR spectroscopic imaging, providing a two-dimensional map across the cross section of hair. And, fi nally, we followed the condition of hair proteins in the amorphous matrix by measuring Td of the α-helical, crystalline phase proteins. All of this information allows us to depict a better picture of how synthetic dyes leach from the hair fi ber. DYE LEACHING KINETICS OF DIFFERENT TYPES OF HAIR Figure 1 presents typical kinetics curves corresponding to dye leaching that were acquired from dyed dark brown and white hair. Dye leaching occurs once dyed hair fi bers come in contact with water. Hair dye loss rates can be determined from the slopes of the curves. By calculation, the dye loss rate within the fi rst ~3–5 min is generally 5–8 times faster than in the later stages, and the dye loss in the fi rst 3 min is ~35% of the total dye loss (within 30 min). More than likely, the dye loss in the early stages of this process corresponds to dye migration from the cuticle region to the solution phase since this is the shortest diffusion route from within the hair structure. Another factor associated with relatively Figure 1. Plots of leached dye from pigmented and nonpigmented hair fi bers as a function of time. The dye concentration in solution was determined by measuring the absorbance at 490 nm. The amount of the dye leaching from hair was calculated from the ratio of the absorbance to the weight of hair tress.
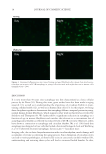
JOURNAL OF COSMETIC SCIENCE 6 quick dye loss in the early stages is the high pH in the dyed hair fi ber. Dyeing hair results in a large increase in pH to ~9. A 2-min rinsing step after the hair dyeing procedure is not suffi cient enough to lower the pH of hair to 6.5. Under high pH conditions, the cu- ticles are swelled and therefore provide open channels and pathways for dye to leach out of the fi bers. It should be noted that most contemporary hair dye systems contain a con- ditioning step to bring the pH down closer to physiological conditions. To probe dye spatial distribution inside the hair, dye species were imaged across hair sections by FT-IR spectroscopic imaging. Figure 2 presents the relative dye concentra- tion across hair sections. The image was generated by integration ratio of the peak at 1116 cm-1 resulting from the dye species to the amide I peak at 1650 cm-1 due to hair proteins. Such an analysis indicates that the cuticle region has higher dye concentrations than the cortex. Free dyes/residues were also visualized in the cuticle areas of the freshly dyed hair fi ber surface (Figure 3) with an optical microscope. Studies have shown that the preferred route of reagents to enter the hair fi ber is the scale edge between cuticle cells, either through the cell membrane complex or endocuticle (11). During the dyeing process, dye molecules aggregate in the cuticle area as reagents perme- ate into the hair fi ber. Therefore, the cuticle area is exposed to and contains a higher amount of dye molecules than other areas of the hair fi ber. These results explain our ob- servations from the kinetics measurements, which indicate that dye leaching is much faster in the early stages immediately after dye deposition. Much of the initial dye fading results from dye loss from the cuticle structure, which is dissolved once the dyed hair fi ber comes in contact with water. Previous work in this area demonstrated that, in addition to the cortex, reactions between the dyes and developer also occur at the surface of hair and in the cuticle (12). In addition, studies by Chandrashekara and Ranganathaiah revealed that dyes diffuse much more quickly into the cuticle than they do into the cortex (13). Therefore, it should stand to reason that since a signifi cant quantity of dyes are located in the cuticle structure, these are likely to diffuse from the hair structure fi rst. As already noted, most contemporary hair dye protocols involve rinsing, shampooing, and conditioning steps to bring the pH of hair down closer to physiological conditions Figure 2. FT-IR image of dye distribution in hair fi ber cross sections. The image was generated by taking the ratio of the integration of the peak at 1116 cm-1 resulting from the dye species to the amide I peak at 1650 cm-1 resulting from hair proteins.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)