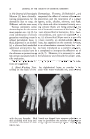
22 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS •t. Radioisotope Studies with Silicone C-l•t (11). In view of the quick dissipation of energy of C-14 beta, one cannot rely entirely on measure- ments made over the skin surface, although, if all physical conditions are constant, an experienced investigator can compare the afforded protection of two or several products with a reasonable degree of reproducibility. For the most accurate determination, it is necessary to excise a small section of skin of a given size, cornbust this, and determine the activity in the evolved carbon dioxide through precipitation as barium carbonate, counting in a flow gas counter or equivalent. 5. Clinical Protection against Contact Dermatitis by patients with dermatitis venenata due to soaps, alkalies, detergents, and household and industrial contactants. Patients are diagnosed as dermatitis venenata particularly of the hands. The majority of the patients are patch tested and the eczematous-producing allergen determined. The patient is then treated with antiseptic sgak s, soothing ointment, and superficial x-ray ther- apy when necessary. Th• pgtient is then advised to use the silicone formu- lation two to three times a day. The patient is requested to gradually cease using the protective rubber gloves with cotton insert. If the physiology of the skin of the hands returns to normal, then household and/or industrial chores are continued. Birmingham (15) re-emphasizes that prolonged action of alkali agents in soaps and detergents tends to neutralize the acid mantle of the skin. They also break down the keratin, to release sulfhydryl groupings. With con- tinuous exposure to the alkali the restoration of the acid mantle is inhibited, and thus the epidermal defenses against allergenic agents is weakened. Anderson (16) and Sharlit (17) offer some valuable and original ideas re- garding the protective action of the acid medium of the skin. The lipid content of the skin also acts as a protective barrier. After many trials, it was decided to disperse various viscosities of the Velvasil fluids in a greaseless base which resembles the pH of the skin, namely around 5.0, to restore and maintain the acid mantle. The viscosi- ties (in centistokes) which were evaluated were 100, 1000, 10,000, and 100,- 000. The concentrations of the silicones were 20, 10, 5, and 2 per cent. The alcohol-soluble Velvasil was dispersed in a homogeneous neutral emul- sion because of its possible cosmetic applicability. The following formulas were utilizect in this evaluation: A. Tween 60 ....................................... 2.0 Citric Acid ..................................... 2.0 Velvasil Silicone Fluid ............................ 10.0 Hydrophilic Ointment (U.S.P.), q. s. ad ............. 100.0 B. Tween 60 ....................................... :2.0 Velvasil Alcohol Soluble Fluid ..................... 10.0 Emulsion Triethanolamine Stearate, q. s. ad ......... 100.0 Forty patients visited our office for dermatological consultation because of a dermatitis involving the hands. These patients presented an erythema-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)