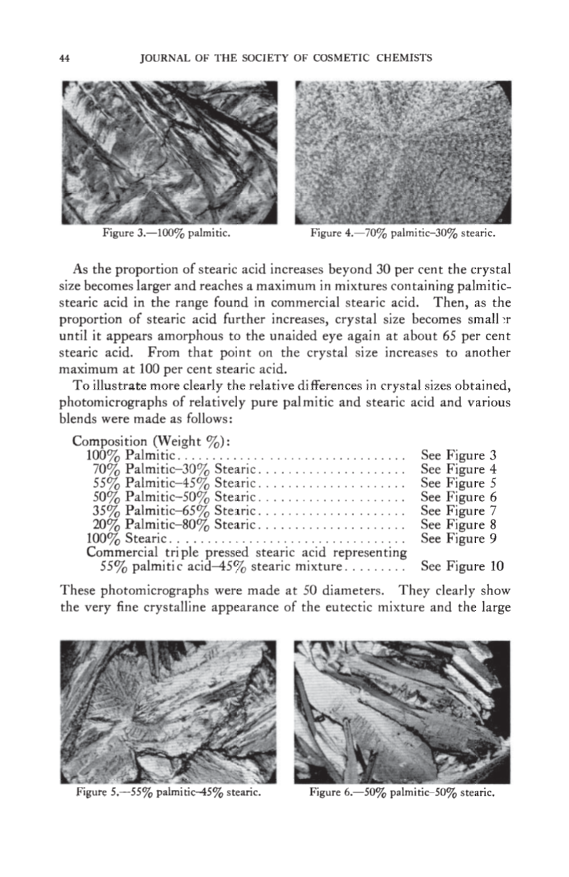
PHYSICAL CHEMICAL PROPERTIES OF STEARIC ACID 43 •0 ß 68 o 66 • 64 • 62 • 6o • 5s [• 56 54 0 10 20 30 40 50 60 70 80 90 100 MOL % STEARIC ACiD Figure 1.--Mol % stearic acid vs. melting point. 0 10 20 30 40 50 60 70 80 90 100 WEIGHT % STEARIC ACID Figure 2.--Weight % steric acid vs. crystal .qlZC. 62.9øC. to about 55øC. This is the point of lowest melting for any mixture of these two fatty acids and is ordinarily referred to as the eutectic. As the stearic acid proportion is increased to about 50 mol per cent, the melting point gradually rises to about 58øC. Commercial stearic acid containing 55 per cent palmitic-45 per cent stearic (weight basis) falls in this section of the curve. On a mol basis this is equivalent to about 57.5 per cent palmitic-42.5 per cent stearic as there is only about 10 per cent difference in molecular weight between these two fatty acids. As the proportion of stearic acid is increased from 50 to 100 mol per cent, the melting point increases sharply to 69.9øC. From this curve it is apparent that mixtures in the range of approximately 70 per cent palmitic-30 per cent stearic acid to 50 per cent palmitic-50 per cent stearic (tool per cent) have properties different from those of mixtures containing higher or lower percentages. Other physical-chemical measurements are of interest and serve to sup- port the evidence obtained from the melting-point curves. SIZE OF CRYSTALS Pure palmitic and stearic acids have comparatively large-sized crystals. Under a given set of conditions, crystals of palmitic acid are larger than those of stearic acid. The relationship between crystal size of mixtures containing various proportions of palmitic and stearic acid is shown in Figure 2, "Weight Per Cent Stearic Acid rs. Crystal Size." Crystal sizes are given qualitatively as they were not determined by actual measure- ment. As the proportion of stearic acid in palmitic is increased from 0 to about 30 per cent (weight) the crystal size becomes smaller. At the eutectic they are so small they appear amorphous or noncrystalline to the unaided eye.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)