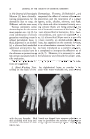
DEVELOPMENT OF AN ECZEMATOUS SENSITIZATION 39 proximity to the area in which these cells have escaped, there would be further interaction as the enzymatic adaptation in the cells in question has caused these cells to react in a different fashion when they again encounter the antigen to which they have become adapted. This further action would then be the basis of the eczematous alterations. In view of the fact that the essential lesion of the eczematous response is a primordial vesicle, it is reasonable to believe that a pyruvate-oxidase system may have been affected, inasmuch as this system is known to be affected in vesicular lesions caused by primary vesiculating agents. According to Andrew and Andrew (6), lymphocytes continuously invade the epidermis and there become transformed into epidermal cells. Un- fortunately, most anatomists and pathologists are in opposition to this theory it is, nevertheless, an extremely attractive suggestion from the point of view of pathogenesis of the eczematous lesions, for if it were true and the lymphocytes that invaded the epidermis and which became trans- formed into epidermal cells were descendants of cells with a specifically adapted enzyme system, it is easy to see how the epidermis in such persons acquired a specific sensitivity toward the antigen in question. For the epidermal cells right at the site of the formation of the antigenic conjugate within the epidermis would be specifically sensitized and consequently capable of reacting with the conjugate in question. BIBLIOGRAPHY (1) Landsteiner, K., and S. Jacobs, John, "Studies on the Sensitization of Animals with Simple Chemical Compound," II, 5 t. Exp. Meal., 64, 625 (October 1, 1936). (2) Gell, P. G. H., Harington, C. R., and R. P. Rivers, "The Antigenic Function of Simple Chemical Compounds: Production of Precipitin in Rabbits," Brit. 7. Exp. Path., 27, 267 (1946). (3) Cullumbine, H., "The Mode of Penetration of the Skin by Mustard Gas," Brit. 7. Dermatol & Syphilol., 58, 291 (1946). (4) Burner, F. M.,"The Edward Stirling Lectures. I. The Basis of Allergic Disease," Meal. 7. •lustralia, 1, 29 (January 10, 1948). (5) Rostenberg, A., Jr., and Brunner, M. J., "Remarks on the Theories of Antibody Forma- tion," •lnn. of •lllergy, 8, 108 (January-February, 1950). (6) Andrew, W., and Andrew, N. V., "Lymphocytes in the Normal Epidermis of the Rat and of Man," •.n_•t: Record, 104, 217 (1949). MEETING MAY 12, 1955 "Topical Uses of Antibiotics: Vehicles Employed," William B. Baker, S. B. Penick and Company "Acetylated Lanolin Derivatives," Lester I. Conrad, American Cholesterol Products, Inc. "Foam Transition and Foam Persistence," M. B. Epstein, Onyx Oil and Chemical Company "Film Properties and Compound Formation in the Sodium Lauryl Sulfate-Lauryl Alcohol-Water System," A. Wilson, Colgate-Palmolive Company 'Symposium on Skin Geriatrics "The Social and Economic Aspects of Skin Geriatrics," E. Anderson, Schering Corp. "The Anatomy and Histology of Aging Skin," Warren Andrew, Wake Forest College "The Chemistry of Aging Skin," Peter Flesh, University of Pennsylvania "The Structural Proteins in the Epidermis and Their Relation to Aging Skin," C. Carruthers, State of New York Department of Health
SOME PHYSICAL CHEMICAL PROPERTIES OF STEARIC ACID* By C. C. TILLOTSON The Procter & Gamble Company, Cincinnati 17, Ohio IT IS WELL KNOWN that the most widely used stearic acid of com- merce is not pure stearic acid. It is a mixture consisting of approximately 55 per cent palmitic acid and 45 per cent stearic acid. Also, it contains other fatty acids primarily oleic, linoleic and myristic in amounts varying with the grade and manufacturing method used. This ratio of palmitic to stearic acid was not originally arrived at through experimentation and selection but was provided by nature in tallow and used because the early methods of manufacturing resulted in this particular combination of fatty acids. A typical composition of fatty acids found in tallow is: Myristic ...................................... 2.0% Palmitic ...................................... 28.0 Stearic ....................................... 21.0 Oleic ......................................... 45.0 Linoleic ...................................... 4.0 Grease is also a source of commercial stearic acid. However, it contains a greater proportion of pahnitic to stearic acid than tallow and also con- tains a somewhat higher percentage of oleic and linoleic acids. For clarity of definition, the term "commercial stearic acid" will be used when referring to the combination of approximately 55/45 palmitic to stearic acid. The term "stearic acid" then will mean the saturated fatty acid, C•7H35COOH. Before discussing some of the physical chemical properties of stearic acid and their importance in cosmetic formulations, it would be well to consider briefly the methods of manufacture for the various types of stearic acid available. 1. Fat Splitting. All oils and fats used to manufacture fatty acids first go through a saponification or fat-splitting process which may be done by * Presented at the December 9, 1954, Meeting, New York City. 4O
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)