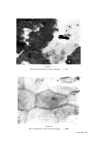
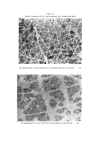
ADHESIVE-TAPE STRIPPING TECHNI•)UE FOR EPIDERMAL HISTOLOGY 461 C/ENERAL DISCUSSION Tape stripping provides a simple method for painless superficial biopsy, to any depth down to granulosum. Its limitations are largely due to the hindrance that can be caused by hair, although this is often not a severe handicap, and to the variability of its results, in terms of evenness of adhesion and depth of stripping. However potentially useful it is as a technique for providing samples of superficial cells, it can only become useful when one has a satisfactory method for examining the samples. The method that we have described is simple and relatively rapid. It involves less processing time than the production of routine paraffin sections, and can provide more information about cells of the skin surface than vertical sections. It is true that the possibility exists that cells may be lost during transference (5), but we have never been able to detect significant cell loss. Goldschmidt and Kligman (5) advocate the use of a glass slide coated with adhesive instead of tape, to reduce the risk of cell loss. But a flexible tape is likely to make better contact with the skin surface and should provide a truer sample of the surface structure. With an adhesive coating on the slide there may be some restriction on the processing methods that can be used. Some stains may colour the adhesive, and some solvents may remove some of the adhesive and cells. We have not tried Wolf's method. It seems possible that there may be greater risk of cell loss by applying tapes to slides coated with xylene and ether, than by putting them on to albuminised slides and getting them well stuck to the slide during incubation before attempting to dissolve the tape's adhesive by soaking in toluene. Keddie's method (4) resembles ours in principle, but albumin seems preferable to gelatin as it is less likely to provide a stained background to obscure important features. One of the problems of interpretation of tape-strippings is how to relate strips to cell layers. By swelling frozen sections of rat skin with sodium hydroxide, the method described by Christophers and Kligman (10), we were able to determine that the 4-week old rats on which most of this work was done, have a 10 or 12-layer corneum. Yet cells with pycnotic nuclei are sometimes found as early as the 4th strip. Those cells appear to be the youngest corneum cells and they are so few in number that they cannot extend much above the bottom layer of corneum. We must therefore be taking off thicker strips than we had suspected. The strips look very thin, and have a single honeycomb pattern. They look like mono layers, except for the fact that some cells, or groups of cells, are more translucent than
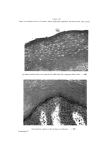
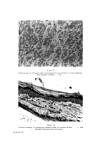
462 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS others. The only suggestion that covers all of these facts is that rat corneum cells are arranged in neat vertical stacks, and that our strips are thicker and more uneven than we suspected. Vertical stacking of corneum cells is sometimes seen in swollen sections of rat skin. Such an arrangement may occur in other species, too. It is a characteristic feature of vertical sections of guinea-pig skin (Fig. 16). The work of Epstein and Maibach (11), and of Porter and Shuster (12) shows that the cells which arise from apparently haphazard mitoses in the basal layer move outwards through the Malpighian layer in a random fashion independently of each other. It is not yet possible to suggest how the mature cells resulting from such a random process can become even partly arranged in neat, vertical stacks. As cells move upwards through the corneum they must undergo some changes. They can hardly achieve their final state in the very abrupt and obvious transition from granulosum cell to corneum cell. In tape-strippings of all the species we have used there is evidence that the youngest cells have pycnotic nuclei, older cells have a clear central space, and oldest cells have no characteristic inclusions. This sequence is readily seen in the thick corneum of human palm and sole. In the upper layers of corneum there can be little chemical activity. Possibly the main change is further dehydration, resulting in shrinkage of the cell, and consequent collapse of the central space (see vertical section of palm - Fig. 10). Goldschmidt and Kligman (5) also concluded that the terminal cell has no internal structure (except melanin granules). Wolf (2) differs from this point of view in two res- pects. He found several sorts of granules in the mature corneum cell, stained by the gram method. We have made little use of the gram stain on tape-strippings so have nothing significant to add on that point. Wolf also concluded that the "nuclear vacuole" represents the maturity of the corneum cells. But we are certain that the clear-centred cell is not quite the terminal cell. It may comprise the bulk of the thick corneum of man, but the superficial cells are without that feature. Wolf (2) used staining intensity, size and type of intracellular granules, and degree of development of "nuclear vacuole", to classify regions of the human body. He separated the zones we have examined into four groups: (1) face and neck, (2) chest, back, stomach, (3) calf, forearm, upper arm, (4) palm, sole, back of hand, instep of foot, shin. Our classification of regions, based on the patterning and groupings of their surface cells, differs from Wolf's in that we do not distinguish back and stomach from upper arm, forearm, and calf we do distinguish back of hand and top of foot from palm and sole we include shin with the trunk, arms and legs group.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)