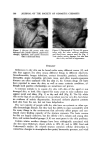
SKIN CONDUCTANCE AND ANTIPERSPIRANTS 339 om•o]sopr'opam]de A ..... Al'annic acid l"lm. m D I'r'ich Ior'a c eric acid ß ß aluminium hydroxychloride X ............ X alumin]urn sulFal,e •._.._& glul,araldehyde I .... I Formaldehyde • . - II I III ] I I II I I1•1 Ill , I ,I I , I I 0.01 0.1 I 10 moles Figure 3. Regression lines "palmar skin conductance variation per cent/concentration" of various antiperspirants Table II Anhidrotic Concentrations 50 and Relative Anhidrotic Activities of Various Antiperspirants Chemicals Weight/Volume (rag/100 ml) Moles x 10 -4 Times of Anhidrotic Relative Anhidrotic Relative Readings Concentration Anhidrotic Concentration Anhidrotic (hours) 50 SE a Activities 50 SE a Activities Tannic acid 4 16,190 1,320 12 951 77 87 7 25,500 1,640 8 1,498 96 55 Trich]oracetic 4 14,770 350 13 9,039 214 9 acid 7 38,900 350 5 23,808 214 3 Aluminum 4 1,990 490 100 '* 824 202 100 b hydroxychloride 7 5•0 566 368 223 231 369 Aluminum sulfate 4 7,430 2,460 27 1,114 369 74 7 Formaldehyde 4 7 51,640 1,810 4 171,961 6,027 0.5 Glutaraldehyde 4 4,390 860 45 4,384 858 19 7 6,360 820 31 6,351 818 13 lsopropamide 4 2,410 96 82 502 20 164 iodide 7 870 70 229 181 14 455 •Standard error of anhidrotie /Sx" (.1•_ concentration 50 equals •v (--50 -- Y? , ¾ n- • x + sy• -- Y is the mean pahnar skin conductance variation per cent. bWeight/Volume/ or molar activity of aluminum hydroxychloride equals 1•.
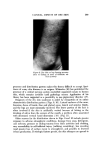
340 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS olum n urn hydroxychloride __ SOmg /100 ml ___ 50mg/100ml (woshing) 0 ]_ • I -I .--- ! ! '•j glutaraldehyde o. .-- 780 mg/100 ml --50 ----7BO mg/100 ml ( washing.1 I I I 1 4 7 hours Figure 4. Effect of washing on palmar skin conductance variation after application of aluminum hydroxychloride and glutaraldehyde at certain concentrations while in the case of glutaraldehyde, strong inhibition of palmar skin conduc- tance could still be detected (pahnar skin conductance variation =- 72.47 per cent. P = 0.05 confidence limits =-59.94 and -85.00 per cent). Persistence of the Anhidrotic Action after Washing At anhidrotic concentration 50 (1,990 and 4,390 mg/100 ml, respectively), the prewashing and postwashing anhodritic activities of aluminum hy- droxychloride and glutaraldehyde remained identical. On the other hand, at minimal active concentrations (50 and 780 rag/100 ml), the topical anhi- drotic action of aluminum hydroxychloride became zero if its application to the skin was immediately followed by washing, whereas, washing did not modify the anhidrotic action of glutaraldehyde (Fig. 4). Discussion The results obtained are consistent with research carried out to date. Trichloracetic acid was seen to be a poor anhidrotic (1) anticholinergic drugs (13, 14) and aldehydes (15, 16) to be active anhidrotics. Glutaralde- hyde had a relatively long effect (15). Aluminum salts revealed themselves as good anhidrotics. Their antiperspirant effect reached a maximum 4 to 7 hours alter application and was still perceptible alter 24 hours (12). Aluminum
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)