j. Cosmet. sci., 49, 1-11 (January/February 1998) Influence o! lameliar liquid crystal structure on percutaneous diffusion o! a hydrophilic tracer from emulsions LAURE BRINON, SANDRINE GEIGER, VALERIE ALARD, JEAN-FRAN(•OIS TRANCHANT, THIERRY POUGET, and GUY COUARRAZE, Laboratoire de Physique Pharmaceutique, URA CNRS 1218, Universitg Paris-Sud, 92290 Chatenay-Mababry, France (L. B., S.G., G.C.), and Laboratoire de Recherche Christian-Dior, 45804 St. Jean de Braye, France (L.B., V.A., J.-F.T., T.P.). Accepted for publication February 1 O, 1998. Synopsis The purpose of this study was to investigate the effect of a lameliar liquid crystalline phase in emulsions on percutaneous diffusion of a hydrophilic sunscreen (benzophenone-4). Six emulsions were formulated, using all the same components except the surfactants. The emulsion structures were visualized by freeze-fracture electron microscopy. In vitro penetration measurements were performed with static diffusion cells. Benzophenone-4 fluxes were smallest for emulsions without liquid crystals and highest for those with liquid crystals. The permeant fluxes seemed to be modified by the surfactant organization within the emulsions. INTRODUCTION Surfactants are major components of cosmetic formulations. They are commonly used to stabilize the emulsions. Indeed, these molecules are able to form a monolayer at the oil-water interface, thus reducing the interfacial forces. Within the concentration range of the emulsifying agents used in topical emulsions, some surfactants may form par- ticular colloidal aggregates such as liquid crystal structures (of lameliar, hexagonal, or cubic type). These lameliar structures, when they surround droplets in an emulsion, can enhance the emulsion stability by preventing droplet coalescence (1). These lameliar structures are also encountered in liposomes. Indeed, a liposome is an aqueous compartment surrounded by one or more lipid bilayers. It has been demon- strated that these particular structures of lipids in the bilayer configuration may enhance the concentration of certain molecules within the skin while reducing their systemic absorption, although the mechanism of action has not yet been fully elucidated (2-5). Many studies have evaluated the influence of different amphipathic compounds on percutaneous absorption (6-11), but generally the authors do not pay attention to the
2 JOURNAL OF COSMETIC SCIENCE vehicle structure. Other studies have investigated the in vitro release of active compo- nents (12-15) and the cutaneous permeability (16-19) as a function of the various colloidal organizations for a given composition of the vehicle. However, in general, effects are demonstrated for one surfactant system. The aim of our study was to evaluate the impact of the presence of lamellar liquid crystals within emulsions on percutaneous permeability for a large variety of surfactants, by comparing six emulsions that only differed by the surfactant used and, as a result, by their structure. CHEMICALS AND FORMULATIONS CHEMICALS The permeant was benzophenone-4 (the sunscreen Uvinul©MS40). A series of six emul- sions containing different surfactants but with the same aqueous and oily phases was prepared. The oily phase was a caprilic/capric triglycerides (Miglyol©812)/octyl methoxycinna- mate (Parsol©MCX) mixture. The surfactants polysorbate 60 (Tween©60) steareth-2 (Brij©72) steareth-21 (Brij©721) poloxamer 407 (Synperonic©PE F/127) sorbitan stea- rate and sucrose cocoate (Ariatone©2121) and triethanolamine stearate were obtained from ICI Americas. The surfactant acrylates/C•o_3o alkyl acrylate crosspolymer (Pemulen©TR1) was obtained from Goodrich. The preservative, diazolidilyl urea (Germall©II), was obtained from Sutton. The sodium hydroxide required for aqueous phase and acrylates/C•o_3o alkyl acrylate crosspolymer neutralization was obtained from Fluka. FORMULATIONS Two hundred grams of each emulsion were prepared using a standardized procedure: surfactant, aqueous phase, and oily phase were weighed into a glass container, sealed, heated to 80øC, and stirred using Istral-type mixing equipment (1050 rpm). Agitation was maintained until the emulsion cooled to room temperature. Three passes through a microfluidizer (Rannie©), set at a pressure of 600 PSI, were necessary to produce stable emulsions with polysorbate 60 and poloxamer 407. The compositions of the formula- tions are listed in Table I. EMULSION STRUCTURE Freeze-fracture electron microscopy was used to study aggregate structures in emulsions with the six different surfactants. The samples were mixed with glycerol (30% of the water content) to prevent ice crystal formation and were rapidly frozen in liquid propane (-196øC). The frozen samples were fractured in a Baizers BAF400 freeze-etching device. Platinium and carbon replicas were made. These solids were evaporated by electron beaguns. Contrast was achieved by depositing the particles of platinium at an angle of 30 ø. The carbon layer was deposited vertically onto the replica. The replica was transferred from one cleaning solution to another. After being washed and dried on an electron microscope grid, the replica was
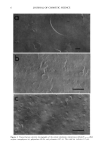
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)