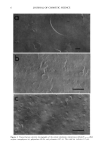
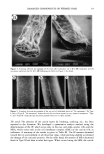
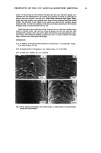
PERCUTANEOUS DIFFUSION OF A HYDROPHILIC SUNSCREEN 7 J/ t / . Figure 3. Freeze-fracture electron micrographs of the structured emulsions containing steareth-2/-21 (a), sorbitan stearate and sucrose cocoate (b), and triethanolamine stearate (c). The scale bar indicates 1 pro.
8 JOURNAL OF COSMETIC SCIENCE Table II The Structural Properties of the Different Emulsions Surfactants Benzophenone-4 Presence of Medium-size partition between lameliar oily droplets oil/water (%) liquid crystals (pm) (w/w) Polysorbate 60 No 0.1 _+ 0.1 21/79 Poloxamer 407 No 0.1 _+ 0.2 8/92 Acrylates/C•o_3o alkyl acryalte crosspolymer No 3.1 -+ 4.6 28/72 So rbitan stearate and sucrose cocoate Yes 10.1 _+ 11.3 21/79 Steareth-2/-21 Yes 6.4 _+ 9.4 25/75 Triethanolamine stearate Yes 0.4 _+ 0.3 11/89 Aqueous reference No --/100 I.N VITRO PERMEATION As benzophenone-4 is a predominantly hydrophilic drug, its permeation from the con- tinuous aqueous phase of emulsion was compared with permeation from an aqueous solution. Figure 4 represents the in vitro permeation profiles of benzophenone-4 from the six emulsions studied and from an aqueous solution as reference (water with 2.5 % benzo- phenone-4). The corresponding steady-state fluxes and the amounts of benzophenone-4 that diffused after 48 h are summarized in Table III. Permeation from the emulsion with artionic surfactant (triethanolamine stearate) was much higher than that from those with nonionic surfactants (the five other surfactants used). This result is in agreement with results concerning artionic and nonionic enhancer permeation effects (20-21). According to the differences obtained between the emulsions and the aqueous solution, the surfactants seemed to have influenced benzophenone-4 transport from the vehicle to the receiver medium. Moreover, the benzophenone-4 fluxes were the smallest for emul- sions without liquid crystals (termed simple emulsions) and the highest for those with liquid crystals (called structured emulsions), and thus the permeant fluxes also seemed to be modified by surfactant organization in emulsions. The overall effect of surfactant on membrane permeability is the result of two opposing events (22-23): on one hand, interaction with cutaneous membrane enhances the per- meation, whereas, on the other hand, association of the permeant molecules with the surfactant into micelie-like structures increases permeant solubility in the vehicle, and so its partition coefficient towards the skin is reduced and permeability decreases (21,24-26). The flux from the simple emulsion with poloxamer 407 was significantly lower than that of the aqueous reference (Student's t-test, p -- 0.05%). For emulsions containing pol- oxamer 407, an interaction of benzophenone-4 with surfactant micelies could be pre- ponderant over a possible interaction of the surfactant with the skin, and this could lead to a decrease in benzophenone-4 permeation. The fluxes from the simple emulsions with polysorbate 60 and with acrylates/C•o_3o alkyl acrylate crosspolymer were smaller than that of the aqueous reference, whereas the flux from structured emulsions with sorbitan stearate and sucrose cocoate was higher,
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)