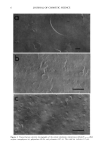
62 JOURNAL OF COSMETIC SCIENCE the elastic forces, i.e. the ratio G"/G', also known as tan delta, is less than 1. The temperature at which tan delta climbs above 1 is the temperature at which phase separation will inevitably occur with time. •n 3 un•tible emul.ion ß ß 1,5 I ß ß ß ß S'•lble ern•,.sl•n I I"l e I1• I" e II ß 0,$ ß eoeooooooo ß ß 0 ß ' ß ß - O 10 20 30 40 • T.mpemtum [ 'C ] O/W-Emulsion K• •T- AK •c..•u'cra• sys•.m to dc•'mmg c,m•b•uY•/ ia m•-m-• Conductivi _ty Methods ff phase separation is occurring in an emulsion, differences in conductivity between the bottom and the top of the body of the emulsion will be detectable considerably in advance of any visual signs of phase separation. This method is applicable to both oil-in-water and water-in-oil systems. Experiments The above principles have been exhaustively tested on a large number of systems. However, we show some exemplary data below to illustrate the principles in operation. An oil-in-water formulation was stabilized by gradually increasing the concentration of a carbomer in the aqueous phase. For each sample, stability was assessed by: • observation of phase separation after storage for 6 weeks at temperature measurement of tan delta between 0 and 60øC 4 hours after emulsion preparation ß observation of the difference in conductivity between top and bottom of the emulsion while cycling between 0 and 60øC over a 24 hour period starting 24 hours after emulsion preparation. INCl Name wløk Olyceryl stearate (and) PEG-100 stearate 2.0• Caprylic/capric triglyceride 8.0• Mineral oil 4.0• Carbomer 0.05, 0.10, 'i3:2oe Glycerin 5.0( Phenoxyethanol 0.5oe Purified Water q.s. 100.0• 0.05% Carbomer 25 deg C 40 deg C 50 deg C , Water Separation 15% vol 40% vol 60% vol Oil Separation no no no ]'an Delta 11.5 2.9 3.2 Difference in Conductivity 0.6 0.6 0.63 0.10% Carbomer 25 deg C 40 deg C 50 deg C Water Separation no no 4% vol [::)il Separation no no no Tan Delta 0.35 0.58 2.2 Difference in Conductivity 0 0 0.26
PREPRINTS OF THE 1997 ANNUAL SCIENTIFIC MEETING 63 0.20% Carbomer 25 deg C 40 deg C 50 deg C Water Separation no no no Oil Separation no no no Tan Delta' 0.31 0.38 0.52 Difference in Conductivity 0 0 0 TESTING SKIN CARE AND COSMETIC PRODUCTS AT HIGH RESOLUTION TO ESTABLISH COMFORT K. Marenus, D. Maes, N. Muizzuddin, *T. Stoudemeyer and *A. Kligman Esteb Lauder Research and Development, Melville, NY, 11746 *Skin Inc., Conshohocken, PA Introduction: In recent years the consumer has come to expect complete comfort from skin care and cosmetic products. Gone are the days when any degree of stinging or irritation are tolerated. In order to meet this challenge, testing far beyond traditional patch tests have been developed. Traditional patch testing, such as the cumulative irritancy test, was adequate in the past but now since the standard of comfort is higher and the products are better, more rigorous tests are required to uncover even the smallest potential for i•xitancy. This new generation of high resolution irritancy testing involves challenging the skin, either physically or chemically before or after a product use period. By either abrogating the barrier or pre-activating the skin's immune machinery, it is possible to evaluate the potential for irritancy or discomfort at a more sensitive level. Methods: Tests for in-itancy at higher resolution involve several different paradigms: In one instance, panelist are asked to use the product for a defined period. At the end of the use period, the skin is challenged, either physically or chemically to determine the integrity of the stratum comeurn barrier. The physical challenge involves using tape stripping to determine the resistance of the barrier with respect to transepidermal water loss. The chemical challenge involves application of an irritant and recording the neurosensory and erythemic responses. Validation of the methods was achieved by evaluating agents known to damage the barrier and create irritancy. Significant correlation was observed between panelist perception objectively measured endpoints.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)