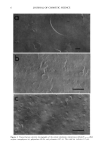
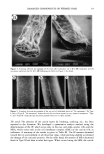
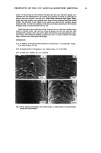
20 JOURNAL OF COSMETIC SCIENCE Table II Changes in the Contents of Each Fraction Toward the Tip End a Length Hair type (cm) Matrix Microfibril HMW Residue + cuticle No treatment 0-10 28.2 _+ 2.3 b 43.4 _+ 3.3 6.9 -+ 1.3 10-20 26.1 _+ 2.0 46.0 _+ 2.8 6.6 _+ 0.7 20-30 24.6 _+ 1.4 45.9 _+ 3.1 7.9 -+ 0.5 30--40 22.6 _+ 2.6 43.6 _+ 4.4 8.3 _+ 2.3 Type I 0-10 26.1 _+ 0.9 44.7 _+ 3.0 6.5 -+ 1.9 10-20 23.6 _+ 1.3 42.4 _+ 5.3 8.5 _+ 2.0 20-30 23.9 -+ 2.3 33.3 -+ 3.3 12.3 _+ 3.3 30--40 22.4 _+ 1.1 24.0 _+ 4.2 18.1 _+ 3.9 Type II 0-10 25.7 _+ 1.2 46.3 _+ 3.9 9.7 -+ 1.9 10-20 22.9 _+ 2.3 45.8 _+ 2.8 10.4 _+ 2.4 20-30 23.4 _+ 2.5 39.2 -+ 3.3 13.9 -+ 3.1 30--40 21.6 _+ 3.3 26.9 -+ 5.3 8.2 _+ 0.3 21.5 _+ 1.7 21.4 _+ 1.6 21.6 _+ 2.2 25.5 _+ 2.9 22.7 _+ 2.0 25.5 _+ 1.4 30.5 _+ 1.7 35.5 -+ 3.4 18.3 _+ 1.8 21.0 _+ 1.4 23.4 _+ 2.6 33.3 -+ 2.5 All values are the mean of three experiments. Refer a Expressed as w/w% of the total recovery. b_+SD. to Figure 1 for experimental conditions. Table III Changes in the Amount of IP and MEA Toward the Tip End Length Hair type (cm) IP • MEA 3 No treatment 0-10 1.1 _+ 0.18 c 56 -+ 3.9 10-20 1.0 _+ 0.12 42 _+ 4.5 20-30 1.0 _+ 0.12 38 _+ 4.4 30•40 0.9 _+ 0.23 36 _+ 5.6 Type I 0-10 1.2 _+ 0.02 52 _+ 1.9 10-20 0.8 _+ 0.14 42 _+ 3.3 20-30 0.7 _+ 0.19 41 _+ 5.2 30--40 0.4 _+ 0.15 3 ! -+ 3.4 Type II 0-10 0.7 _+ 0.22 56 _+ 3.8 10-20 0.7 _+ 0.13 29 _+ 3.3 20-30 0.5 _+ 0.19 17 _+ 1.7 30--40 0.2 _+ 0.19 12 _+ 2.2 All values are the mean of three experiments. Refer to Materials and Methods for experimental conditions. • Isopeptide is expressed as residues/1000 amino acids of hair. a 18-methyl-eicosanoic acid is expressed as micrograms/100 mg hair. c_+ SD. layers of the Type II hair were significantly abraded (Figure 5c). It was reported that the CMC plays a role in intercellular adhesion. The split-end hair was not so damaged on the CMC, and the cuticle layers may remain (Figure 5b). The oxidation of each component. Cysteic acid is the major product of cystine oxidation due to various causes, and many investigators have studied the changes in cysteic acid content. Our method permitted the analysis of the oxidation of each component. Tables IV and V show the decrease in cystine and the increase in cysteic acid. It has been
DAMAGED COMPONENTS OF PERMED HAIR 21 Table IV Changes in the Half-Cystine Contents of Each Fraction Toward the Tip End • Length Hair type (cm) Matrix Microfibril Residue + cuticle No treatment 0-10 25.0 + 4.5 b 9.2 _+ 3.2 10-20 23.6 -+ 2.9 9.2 -+ 2.9 20-30 22.4 + 3.1 10.0 + 3.0 30•40 22.4 _+ 3.3 9.7 + 1.8 Type I 0-10 25.4 _+ 5.5 10.8 _+ 2.3 10-20 22.4 _+ 5.5 7.2 + 4.5 20-30 21.2 _+ 6.0 6.6 + 5.1 30-40 20.1 + 2.5 6.0 + 6.2 Type II 0-10 20.2 _+ 4.9 11.9 + 3.7 10-20 19.8 + 4.1 11.6 + 2.7 20-30 18.9 + 3.9 11.4 _+ 4.2 30-40 18.9 -+ 3.6 11.5 + 5.7 16.4 _+ 3.3 15.6 + 4.2 15.2 +_ 5.8 15.1 _+ 4.9 15.8 + 3.8 15.5 _+ 2.9 12.7 + 5.3 10.0 + 4.7 19.5 -+ 4.1 16.8 ñ 4.4 15.8 + 6.7 10.4 + 4.6 All values are the mean of three experiments. Refer to Figure 1 for experimental conditions. • Estimated as cysteic acid and expressed as residues/100 amino acids. b+SD. reported that the disulfide bond reformation with peroxide following perming is not complete and that the cysteic acid content in the perreed hair is higher than that in untreated hair. Our results showed similar concerns with the Type I hair, but the Type II hair showed an interesting result: although the residue (including the cuticle) was also oxidized just as in Type I, the cortical protein was not oxidized like the untreated hair. In other words, in the split-end hair, not only the cuticle but also the cortical proteins were oxidized. Table V Changes in the Cysteic Acid Contents of Each Fraction Toward the Tip End • Length Hair type (cm) Matrix Microfibril Residue + cuticle No treatment 0-10 3.7 + 0.33 2.5 + 0.1 10-20 4.5 + 0.4 3.0 _+ 0.9 20-30 5.3 + 1.1 3.3 + 0.6 30-40 7.2 + 0.9 4.6 + 0.5 Type I 0-10 3.2 -+ 1.2 2.6 _+ 1.1 10-20 8.4 + 2.1 6.7 + 1.3 20-30 16.3 + 1.3 12.3 + 0.8 30-40 17.5 _+ 0.9 14.5 + 2.4 Type II 0-10 1.6 + 1.5 0.2 + 0.2 10-20 2.7 _+ 0.7 1.4 + 0.8 20-30 4.5 + 0.6 2.4 _+ 0.3 30-40 6.3 + 2.7 2.8 + 1.1 4.2 + 0.3 4.3 + 0.6 4.9 + 0.6 6.3 -+ 1.0 4.3 + 7.5 + 11.7 + 13.1 + 4.7 + 8.8 _+ 12.4 _+ 13.0 + 0.8 1.2 0.7 3.4 2.4 1.3 4.4 2.9 All values are the mean of three experiments. Refer to Figure 1 for experimental conditions. • Expressed as residues/1000 amino acids. •+SD.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)