14 JOURNAL OF COSMETIC SCIENCE how the hair components, especially proteinous molecules, change due to perming. Generally, the hair proteins are extracted by reductants and denaturing agents such as urea or guanidine. Further isolation of the main classes of hair proteins (the matrix and the microfibril) requires an isoelectric precipitation step or electrophoretic method (9). This method is not of practical use for the analysis of individual hairs because it requires many hairs and is a complicated method. Thus, we attempted to develop a simple and easy extraction method for quantifying hair components using a reductant with an anionic surfactant. The goal of this study is the analysis of damage to an individual hair due to perming. MATERIALS AND METHODS CHEMICALS Analytical grade 2-mercaptoethanol (2-ME), dithiothreitol (DTT), tricine, and SDS were supplied by Nacalai Tesque, Inc. (Kyoto, Japan). 4-Bromomethyl-7-methoxycoumarin was purchased from Dojindo Laboratories, Inc. (Kumamoto, Japan). •/-Glutamyl-g- lysine (isopeptide), pronase, leu-aminopeptidase, prolidase, and carboxypeptidases were purchased from the Sigma Chemical Co. (St. Louis, MO). HAIRS For the development of the methods, we collected the root end hair from ten Japanese women who had not been exposed to chemical treatments such as perming or dyeing. Hair was washed using 1% (w/w) SDS and then thoroughly rinsed with tap water. The hair was dried, and external lipids were extracted for 16 hours at room temperature in a 100-fold solution of chloroform/methanol (2:1 v/v). The delipidized hair was cut into 10-mm sections. For the analysis of an individual hair, we collected 100 fibers ofpermed hair from two Japanese women who had permed every two or three months. One hundred hairs that had not been exposed to cosmetic treatments such as perming or dyeing were also collected from one Japanese woman. The hair was cut into 100-mm lengths from the root end to the tip end and then treated as described. METHODS Extraction of hair proteins. The hair proteins were extracted using an anionic surfactant with reductant. In detail, a 10-mg sample of delipidized hair was immersed in 1 ml of 25 mM Tris-HC1 buffer (pH 8.3) that contained 1% SDS and various concentrations of 2-ME. The protein was extracted for three days at 50øC. The extracted protein was analyzed using Tricine-SDS-PAGE (10). Fractionation of hair components. The hair components were fractionated according to the scheme illustrated in Figure 1. A 10-mg sample of delipidized hair was immersed in a 1-ml solution of 25 mM Tris-HC1 buffer (pH 8.3) that contained 1% SDS and 2 M 2-ME. The matrix protein was extracted for three days at 50øC. The hair was washed with 25 mM Tris-HCl buffer, then immersed in a 1-ml solution of 25 mM Tris-HC1 buffer (pH 8.3) that contained 1% SDS and 0.4 M 2-ME. The microfibril protein was
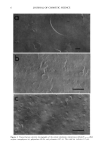
DAMAGED COMPONENTS OF PERMED HAIR 15 Hair CHCI3/MeOH, 16 hours at room temp. I I 1N NaOH/90% MeOH, 2 hours at 60 øC 1% SDS/2M 2-ME, 3 days at 50 øC 1 Integral lipids 1% SDS/0.4M 2-ME, 3 days at 50 øC 1%SDS/0.2M DTT, 6 days at 37 øC r ............................................................[•,, [ [ II-IighmolulaWight i I Cuti'+sidu .. ................................. Cortex ........................................ : Figure 1. Scheme for fractionation of hair components. A 10-mg hair was used in this work, with liquor ratio at 100. See Materials and Methods for details. extracted for three days at 50øC. The residue was removed and washed with water, then lyophilized and weighed. Furthermore, the residue was extracted with a 1-ml solution of 25 mM Tris-HC1 buffer (pH 8.3) that contained 1% SDS and 0.2 M DTT for six days at 37øC to extract the high-molecular-weight protein. After washing and lyophilization, the residue was weighed again. The loss in weight was regarded as the high-molecular- weight protein. The residue included the cuticle. The amounts of the matrix and microfibril protein were determined using the Bio-Rad protein assay (Bio-Rad Lab., Hercules, CA) and compared with a protein calibration curve of BSA in the extraction buffer. Characterization of the extracted fractions. Each extracted fraction was confirmed by amino acid composition and molecular weight. The molecular weight of the extracted protein was determined using Tricine-SDS-PAGE (10). The amino acid analysis was performed according to the Pico-Tag © method supplied by Millipore Co. (Milford, MA). The half-cystine content was estimated as cysteic acid converted by performic acid. Determination of the isopeptide (IP). This method was previously published by Adamski (11). Isopeptide was determined by amino acid analysis after successive peptidase di- gestion. Determination of 18-methyl-eicosanoic acid (MEA). 18-Methyl-eicosanoic acid, which exists only in the cuticle, was analyzed by the method of Wertz (12) with slight modification. Briefly, 10 mg of delipidized hair was hydrolyzed with 1 N sodium hydroxide/90%
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)