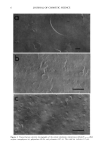
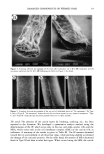
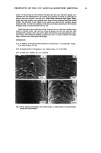
22 JOURNAL OF COSMETIC SCIENCE CONCLUSION We have developed a novel method for quantifying hair components. This method permits the detailed analysis of components damaged due to perming. In summary, we have found a significant decrease in microfibril protein and an increase in high- molecular-weight protein on the tip end of permed hair. We suggest that the "intact" microfibril protein turns into high-molecular-weight protein due to perming. Moreover, we have found that the cuticle was not so damaged, though the cortical proteins were oxidized in the split-end hair. Using this method, additional work is currently being undertaken in order to ascertain the correlation between the morphological changes and the degree of damage. REFERENCES (1) C. M. Pande and J. Jachowicz, Hair photodamage--Measurement and prevention,J. Soc. Cosmet. Chem., 44, 109-122 (1993). (2) S. E. Kelly and V. N. E. Robbinson, The effect of grooming on the cuticle, J. Soc. Cosmet. Chem., 33, 203-215 (1982). (3) J. A. Swift and A. C. Brown, The critical determination of the fine changes in the surface architecture of human hair due to cosmetic treatment, J. Soc. Cosmet. Chem., 23, 695-702 (1972). (4) C. R. Robbins, Chemical and Physical Behavior of Human Hair, 3rd ed. (Springer-Verlag, New York, 1994), pp. 211-226. (5) S. H. Bong and H. Zahn, Contributions to the chemistry of human hair. II. Lipid chemical aspects of permanently waved hair, Int, J. Cosmet. Sci., 11, 167-174 (1989). (6) J. Chao, A. E. Newsom, I. M. Wainwright, and R. A. Mathews, Comparisons of the effects of some reactive chemicals on the proteins of whole hair, cuticle and cortex,J. Soc. Cosmet. Chem., 30, 401-413 (1979). (7) R. C. Marshall and J. M. Gillespie, Comparison of samples of human hair by two dimensional elec- trophoresis,J. Forensic. Sci. Soc., 22, 377-388 (1982). (8) C. Nappe and M. Kermici, Electrophoretic analysis of alkylated proteins of human hair from various ethnic groups,J. Soc. Cosmet. Chem., 40, 91-99 (1989). (9) J. M. Gillespie, "The Structure Proteins of Hair: Isolation, Characterization, and Regulation of Bio- synthesis" in Biochemistry and Physiology of the Skin, L. A. Goldsmith, Ed. (Oxford University Press, London, 1983), Vol. 1, pp. 475-510. (10) H. Shiigger and G. U. Jagow, Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa, Anal. Biochem., 166, 368-379 (1987). (11) J. Adamski, B. Husen, H. H. Thole, U. G. Stewart, and P. W. Jungblut, Linkage of 1713-oestradiol dehydrogenase to actin by e-(y-glutamyl)-lysine in porcine endometrial cells, Biochem. J., 296, 797- 802 (1993). (12) P. W. Wertz and D. T. Downing, Integral lipids of human hair, Lipids, 23, 878-881 (1988). (13) W. Diinges, 4-Bromomethyl-7-methoxycoumarine as a new fluorescence label for fatty acids, Anal. Chem., 49, 442-445 (1977). (14) J. A. Swift, Chemical composition of various morphological components isolated from human hair cuticle, Cosmet. Toiletr., 91, 46-48 (1976). (15) J. A. Swift and B. Bews, The chemistry of human hair cuticle. I. A new method for the physical isolation of cuticle. J. Soc. Cosmet. Chem., 25, 13-22 (1974). (16) A. Nakamura, R. Kon, and K. Takeuchi,Japanese Patent (submitted). (17) H. Zahn, Wool chemistry and processing, Abstracts of Proc. 9th Int. Wool Textile Res. Conf (Biella), 1995, pp. 1-16. (18) H. Zahn, Wool is not keratin only, Abstracts ofProc. 6th Int. Wool Textile Res. Conf (Pretoria), 1980, pp. 1-45. (19) N. Yorimoto and S. Naito, Physical and chemical properties of integral lipids in hair cell membrane complex, Proc. ISF '94 (Yokohama), preprint, 1994, p. 215.
j. Cosmet. Sci., 49, 23-32 (January/February 1998) Influence of glycolic acid as a component of different formulations on skin penetration by vitamin A palmitate GISLAINE RICCI LEONARDI and PATRICIA MARIA BERARDO GONOALVES MAIA CAMPOS, Faculty of Pharmaceutical Sciences of Ribeir•o Preto, University of S•o Paulo, Av. do Caf s/n, 14040-903 Ribeir•o Preto, S•o Paulo, Brazil. Accepted for publication February 16, 1998. Synopsis Among the many active agents for dermocosmetic purposes that have been described, marketed, and prescribed, vitamins (vitamin A palmirate among them) and alpha-hydroxy acids such as glycolic acid have been gaining scientific importance. When penetrating the skin, vitamin A palmirate contributes to leaving it soft and smooth, improving its properties as a water barrier. With the topical application of vitamin A palmirate, the skin is stimulated to produce more epidermal protein, making the epidermis thicker and covered with a better formed keratin layer. Glycolic acid is part of a new generation of cosmetics used for treatment. It is a fascinating active agent with a simple molecular structure that has yielded highly satisfactory results in terms of recovery of aged skin. The combination of low concentrations of glycolic acid with vitamin A palmkate has been extensively used in dermocosmetic formulations since it has been speculated that glycolic acid reduces the cohesion of corneocytes, stimulating natural skin desquamation, and also increases skin hydration, thus being considered to increase skin penetration by vitamin A palmirate and to potentiate its pharmacodynamic effects. The objective of the present study was to investigate the influence of the presence of glycolic acid on in vivo skin penetration by vitamin A palmirate. Non-ionic gel, gel cream, and cream formulations containing vitamin A palmirate combined or not combined with glycolic acid were studied. The formulations were applied to delimited areas on the depilated dorsum of 24 guinea pigs, and biopsies were collected one, two, and four hours later to determine the percentage of vitamin A palmirate that penetrated the skin during these time intervals. The results indicate that the presence of glycolic acid in the formulations containing vitamin A palmirate increases the behavior of skin penetration by vitamin A palmirate along time in the gel formulation. INTRODUCTION Among the numerous active compounds used for dermocosmetic purposes that have been described, marketed, and prescribed, vitamins and alpha-hydroxy acids are becom- ing increasingly popular, representing a challenge for many researchers (1-8). Vitamin A in its different forms has been widely used in topical preparations, and its esters are used as components of cosmetic formulations. When absorbed through the skin, vitamin A palmitate contributes to the maintenance of skin softness and smoothness, improving the water barrier properties of this tissue. This property has led to the use of vitamin A palmitate for the treatment of seasonal/environmental problems (dehydration, heating, 23
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)