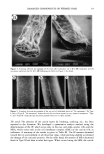
16 JOURNAL OF COSMETIC SCIENCE methanol at 60øC for two hours. The fatty acids liberated with solvent were analyzed by HPLC after being labeled with 4-bromomethyl-7-methoxy-coumarin (13). Analysis of hair from individuals. Hair components were fractionated according to the scheme illustrated in Figure 1 using 10 mg of hair from each of three women. The contents of fractionated components were determined. IP in the residue fraction and the contents of half-cystine and cysteic acid in each fraction were also determined using amino acid analysis. MEA was determined using another 10 mg of hair. Scanning electron microscopy (SEM). Morphological changes were examined using a Hita- chi-S520 scanning electron microscope after sputtering with gold. RESULTS AND DISCUSSION FRACTIONATION OF HAIR COMPONENTS The main classes of hair proteins can be discriminated by their molecular weight. The molecular weight of the matrix protein is in the range from 10 to 30 kDa, and that of the microfibril protein is 45-55 kDa (9). During the extraction of hair constituent protein using an anionic surfactant with reductant, we found that the amounts of extracted proteins were influenced by the concentration of reductant. Figure 2 shows the effect of the concentration of 2-ME on the extraction of the cortical protein. The extraction was maximized in a range between 0.4 M and 0.8 M 2-ME. When the 2-ME concentration was higher, the extracted amount of the microfibril protein was signifi- cantly decreased, and it could not be extracted at the 2M 2-ME level. However, that of the matrix protein did not change very much. Therefore, only the matrix protein was first extracted using 2M 2-ME, and then the microfibril protein could be extracted using 0.4 M 2-ME after the matrix protein had been extracted. The mass of these cortical proteins was about 70% of the hair. We could also extract a small additional amount of protein using DTT, a stronger reductant than 2-ME. The molecular weight of this 97K 67K 43K 31K 22K 14K IlK 6K a b c d e f g Figure 2. Effect of the concentration of 2-ME on the extraction of the cortical protein The cortical protein was extracted for three days at 50øC in 25 mM Tris-HCl buffer (pH 8.3) with 1% SDS and 2-ME. 2-ME concentrations were (b) 0.2 M, (c) 0.4 M, (d) 0.8 M, (e) 1.2 M, (f) 1.5 M, and (g) 2.0 M, respectively. Aliquots were submitted to Tricine-SDS-PAGE. (a) Molecular weight standards.
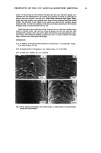
DAMAGED COMPONENTS OF PERMED HAIR 17 protein is more than 100 kDa (Figure 3), and so we called this protein the high-molecular- weight protein (HMW). We confirmed the purity and identity of extracted fractions not only by molecular weight but also by amino acid composition. The amino acid composition of each fraction from the root end hair is summarized in Table I. The extracted protein using 2M 2-ME was in good agreement with the matrix protein, and the 0.4 M extract was the micro- fibril protein when prepared by reduction following alkylation (9). The amino acid composition of HMW, which increased toward the tip end, as discussed later, was similar to that of microfibril protein. The residue was almost the same as the amino acid composition of the cuticle prepared by physical isolation (14,15) in the root end. Figure 3 shows the Tricine-SDS-PAGE of each fraction except the residue. The molecular weight of each fraction was also confirmed, and they showed good purity. When viewed by SEM, the hair from which only matrix protein had been extracted was shriveled but still had a fibrous shape, as seen in Figure 4a. On the other hand, when both the matrix and the microfibril protein were extracted, the hair was no longer fibrous and only the cuticle layers remained just like the sheath (Figure 4b). Conse- quently, we have established a method of fractionating the hair components, as shown in Figure 1. As we have previously described (16), we cotfid not separately extract the matrix and the microfibril protein when the reductant is thioglycolic acid (TGA), and the extracting efficiency is not enough when the surfactant has the properties of low denaturation. We considered that the microfibril protein requires a strong denaturing surfactant for ex- traction since it is a tightly packed structure. Both 2-ME and DTT have alcoholic OH in their molecules, and the denaturing efficiency of the surfactant is weakened if the concentration of these reductants is too high. This is the reason why only the matrix protein was extracted using the concentrated reductant. In fact, the mild surfactants such as sodium dodecyltri (oxyethylene) sulfate cotfid not extract the microfibril protein (16). This is a unique method of isolating the main classes of hair proteins (the matrix and the microfibril). It is an easy, high-yield method that does not need to be modified. In this 97K 67K 43K 31K 22K 14K 11K a b c d 6K Figure 3. Tricine-SDS-PAGE of extracted fractions: (a) molecular weight standards (b) 2 M 2-ME extract (c) 0.4 M 2-ME extract after (b) (d) 0.2 M DTT extract after (c). Refer to Figure 1 for detail.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)