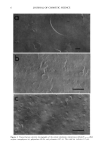
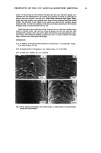
48 JOURNAL OF COSMETIC SCIENCE Figure 3: Interfacial Tension Measurements. Figure 4: Conductivity measurements vs. temperature during the cooling phase of the emulsification process. The newly developed emulsification technology works differently than a soap-based system: the rheology modifier/emulsifier dissolves @80øC •o form a lameliar liquid crystalline phase during cool down, a phase transition occurs between 35-45ø(2, as indicated by the change in conductivity in Figure 4, and the emulsion "sets". The phase change yields a network that provides rigidity at the oil/water interface, preventing EMULSIl•ICATION TECHNOLOGY Prototype formulations based on the newly developed technology will be provided to demonstrate important attributes such as efficiency, versatility and skin aesthetics. Some in-vivo evaluations, such as irritation test results, SPF static and waterproof will also be presented in correlation with in vitro work in support of the superior mildness and SPF efficiency of the emulsions employing the newly developed emulsification systems. The key findings are that the emulsifying system has a synergistic effect with physical sunscreens and water soluble sunscreens in combination with organic sunscreens. An SPF enhancement up to 40% (rs.conventional emulsions employing the same level of sunscreens) was achieved with this technology, as illustrated in Figure 5*. Figure 5: SPF enhanced emulsions vs. conventional emulsions employing the same level of sunscreens Figure 6: Microscopic evaluation of sunscreen emulsion. Figure 6 illustrates the ability of the technology to evenly distribute the physical sunscreens in combination with organic sunscreens in the continuous film resulting in an SPF over 30. Most importantly, the visual consumer signal is positive because no whitening effect upon rub in is noticeable. Possible explanations for the mechanism of emulsification will be presented based on rheological and microscopic evaluation of the emulsions. *Note: Due to lack of space, only a few results are illustrated. More results will be shown in the presentation.
PREPRINTS OF THE 1997 ANNUAL SCIENTIFIC MEETING 49 RHEOLOGY MODIFICATION OF HYDROGEN PEROXIDE-BASED APPLICATIONS USING A CROSS-LINKED POLYACRYLIC ACID POLYMER Julie Schmucker-Castner and Dilip Desai BFGoodrich Company, Brecksville, Ohio 44141 Introduction: Hydrogen peroxide has been widely used as an oxidizer and bleach in hair care applications since the 1940s. Hydrogen peroxide is one of the most important ingredients in almost all hair color and permanent wave products on the market today. It is also used frequently in a variety of topical and oral care applications. As environmental regulations and consumer trends make the use of chlorine bleach less acceptable in detergent and household products, "oxygen" bleach will be used more widely. The focus of thickening hydrogen peroxide in this paper will be for the applications of hair bleach and two-part permanent hair color. Hydrogen peroxide, by nature of its reactive chemistry, is difficult to stabilize in many applications. There are many commonly-used ingredients that are incompatible with hydrogen peroxide. Careful selection of formula ingredients, thickeners, stabilizers, as well as the proper order of addition, pH, and processing equipment are all essential to ensure acceptable long-term physical and chemical stability of a finished product. Thus, achieving both the desired viscosity and appropriate rheological properties can be a challenging task in peroxide-based formulations. Although cross-linked polyacrylic acid polymers are currently being used in some hydrogen peroxide-containing personal care and topical pharmaceutical applications, no systematic studies have been done to evaluate different cross-linked acrylic acid polymers for specific hydrogen peroxide applications and the optimum conditions to achieve acceptable long-term stability. Recent systematic experiments have demonstrated that excellent long-term stability of hydrogen peroxide- based gels, thickened with cross-linked polyacrylic acid polymers, can be achieved. A fundamental study of the stability of hydrogen peroxide-based gels, thickened using cross-linked polyacrylic acid polymers, will be presented. The study evaluates different types of cross-linked polyacrylic acid polymers (as well as an associative acrylic polymer), several commercial sources of hydrogen peroxide, varying hydrogen peroxide concentrations, and the effect ofpH. Viscosity results from some of the combinations of these variables will be presented. The effect of accelerated aging on percent active peroxide and viscosity of the thickened peroxide gels will also be discussed. Materials and Methods: Experiments: Study I: Two separate studies of thickening hydrogen peroxide with cross-linked polyacrylic acid have been performed. The first study evaluated a single source of hydrogen peroxide (peroxide "D" and a single cross-linked polyacrylic acid polymer (Polymer A, fig. 1). Gels were made at four peroxide concentrations (3, 6, 9, 12 % •) and three pH values (3.5, 4.0, 4.5). These low pH values were selected because of the inherent instability of hydrogen peroxide at neutral or high pH. Ammonium hydroxide was used to adjust the pH of the gels. Study II: After learning a great deal from the first study, a second study was initiated. The second study focused on peroxide and pH values appropriate for two-part hair dye applications. Most of the discussion of this presentation will focus on the second study. Four cross-linked acrylic acid polymers, as well as a liquid acrylic associative polymer, were evaluated in the second study. Several commercial sources of hydrogen peroxide were used in the study at a concentration of 6.0 % •t. The gels were made at three pH values: 2.7 (no neutralizer), 3.6, and 4.0. As before, ammonium hydroxide was used to adjust the pH of the gels. Thickening Polymers: The polymers used in the study will be referred to as Polymer A, B, C, D, and E (fig. 1). All polymers were used at 1.0 % •n active solids level in the peroxide gels in both studies. Hydrogen Peroxide: Four types of commercially available hydrogen peroxide were evaluated in the two studies: Peroxide A and C are standard personal care grades, peroxide B and D are specially stabilized grades. They will be referred to in this study as Peroxide A, B, C, and D. Formula and Procedure: A standard 6 % •n active hydrogen peroxide gel formula and preparation procedure were used for both studies. Samples were made in plastic beakers and mixed with plastic stirrers. The test formula was made in the following order
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)