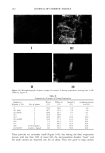
j. Cosmet. sci., 53, 151-164 (May/June 2002) Phase behavior of the •-hydroxyoctanoic acid/Laureth 4/white oil/water system and preliminary evaluation of the phase chanDes durinD evaporation of its emulsion ABEER AL-BAWAB, Chemistry Department/Faculty of Science, University of Jordan, Amman 11942, Jordan, and STIG E. FRIBERG, Center for Advanced Materials Processing and Department of Chemistry, Clarkson University, Potsdam, New York 13699-5814. Accepted for publication January 31, 2002. Synopsis The phase diagram was determined for the o•-hydroxyoctanoic acid/Laureth 4/white oil/water system using visual observation with an optical microscope. Typical emulsions were evaporated to determine the struc- tural changes. These were compared to those predicted from the phase diagram. Small-angie x-ray diffraction was used to determine the location of the o•-hydroxyoctanoic acid in the lameIlar liquid crystal. The most important result is the fact that irrespective of initial composition, the final result was a suspension of solid acid particles with dissolved oil and surfactant in a surfactant/oil liquid with only minute amounts of solubilized acid. INTRODUCTION Alpha-hydroxy acids (AHAs) are fruit acids, which include glycolic (sugar), lactic (milk), malic (apples), and tartaric (grapes) acids. They have become popular as components in skin lotions because they hasten the skin's natural exfoliation process by dissolving the lipid bonds between the skin's surface cells and expediting their removal (1,2). As we age, the exfoliation process tends to slow down and the outer layer (stratum corneum) thickens, causing a rough, dry appearance. With an enhanced rate of exfoliation, younger, plumper-looking cells are exposed. Until recently, studies of (x-hydroxy acids (1,2) have been focused entirely on functional changes in the human stratum corneum induced by the application of (x-hydroxy acids. These studies were concerned with the action of (x-hydroxy acids on the skin, evaluating their relative stimulation and irritancy (1,2). The results of these investigations are mainly related to the effects of the acid per se it is typically applied as an alcoholic solution. These reports, although valuable, do neglect a very essential point. After application of 151
152 JOURNAL OF COSMETIC SCIENCE a skin care formulation, the water and volatile organic compounds are to a very large degree evaporated with 30 minutes. Hence, the activity structure of the formulation is determined by the residue after evaporation of volatile components (3-8). This structure is found in the phase behavior of the system, but, unfortunately, these conditions have not been studied for the ot-hydroxy acids. With this fact in mind, we found a system of ot-hydroxyoctanoic acid, a nonionic surfactant, Laureth 4, white oil, and distilled water to provide useful information about the behavior of the acid after application. White oil was chosen because it is widely used in cosmetics (6). Alpha-hydroxyoctanoic acid was chosen for this investigation, because at 3% this acid showed the highest therapeutic index of all acids in a recent evaluation. In the study, special attention is focused on phase changes, inversion of the emulsion, the position of the ot-hydroxyoctanoic acid during the evaporation process, and the final state of the emulsion. EXPERIMENTAL MATERIALS The following chemicals were used without further purification. Alpha-hydroxyoctanoic acid (99%) was from Sigma Chemical Co., St. Louis, MO polyoxyethylene-4-1auryl ether (Brij 30), Laureth 4, was from ICI Surfactants, Wilmington, DE white oil was from Penreco, Karns City, PA. Water was doubly distilled. All materials were used without further purification. PHASE DIAGRAM DETERMINATION The solubility regions were determined by addition of either water or white oil to combinations of Laureth 4/ot-hydroxyoctanoic acid, noticing the points of clarity and turbidity. The extent of the solubility regions was confirmed at first by centrifugation of samples at 5000 rpm for 30 minutes and, for samples with composition close to the solubility limit, also by storage at room temperature for several days. The liquid crystalline phases were identified by optical microscopy, with the samples between crossed polarizers, and their boundaries were confirmed by the results of small- angle x-ray diffraction based on knick points in the curves of interlayer spacing versus composition. All phase diagrams were determined at room temperature (22 ø + iøC). The three-phase areas were determined by analysis of the composition of each phase in equilibrium. The limit of the phases was determined using the following procedure: A series of samples with different ratios of ot-hydroxyoctanoic acid and surfactant in the two phases was prepared and water was added. The samples were observed under a microscope, between crossed polarizers, and the limit of three-phase areas was detected from the appearance of liquid crystal and the limit of a two-phase area was detected by disappearance of one isotropic phase. Tie lines were determined by preparing a series of samples in the emulsion region, separating them by centrifugation, and then analyzing the separated phases for composition. The two-phase emulsion region of the solid acid and oil/surfactant solution was separated by centrifugation at 5000 rpm for two minutes, and the weight ratio of each phase in equilibrium was taken and compared to those of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)