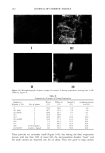
186 JOURNAL OF COSMETIC SCIENCE •' rOH HO'¾ HO b-X 'O•oo • "OH OH Figure 1. Structure of cyclodextrins (dimensions in pro). and very recently textiles are known (12-14). Only a few applications of cyclodextrins in cosmetics have been published (12,13,15-17). Therefore, it seems to be worthwhile to discuss in general possible applications of cyclodextrins in cosmetic products and to give some examples of their present uses. PROPERTIES OF CYCLODEXTRINS AND THEIR COMPLEXES Cyclodextrins are formed during the enzymatic degradation of starch. They are poly- saccharides built from six to eight (o• = 6, ]3 = 7, •/= 8) D-glucose units. The D-glucose units are covalently linked at the carbon atoms C• and C 4. The radii of the rigid cavities vary from 0.50 to 0.85 nm. In these cavities guest molecules can be enclosed. For the complex formation between cyclodextrins and guest molecules, agreement between the size of the cavity and of the guest molecule is not essential. The complex formation takes place only if a part of the guest molecule is located inside the cavity. Due to the complex formation, the physical and chemical properties of the enclosed guest molecules change, e.g., the vapor pressure is reduced and the solubility in water increases. The most important advantages for the use of cyclodextrins in cosmetics are: (1) Protection of the guest molecules against: © decomposition reactions induced by light or heat © oxidation or hydrolysis © chemical reactions with other organic compounds © loss by evaporation (2) Solubilization of the guest molecules in water: © increase of solubility © increase of the rate of solubilization © avoidance of organic solvents © change of viscosity
CYCLODEXTRINS 187 (3) Elimination of: © undesired odors or tastes © hygroscopicity (4) Improvement of handling: © of liquid or oily substances as powders © increase in the stability of emulsions In contrast to starch, cyclodextrins and their derivatives are not a nutrient medium for microorganisms. As a result, the use of preservatives in formulations can be reduced. This is a further advantage of cyclodextrins. Detailed studies on the toxicity, mutagenicity, teratogenicity, and carcinogenicity of cyclodextrins and some of their derivatives have been performed (18). These results indicate that cyclodextrins may be harmful to the human organism only at extremely high concentrations. No indication of acute toxicity was observed during animal ex- periments. Since November 2000, 13-cyclodextrin has been licensed in Germany as an additive in food (19). This had already been done in Japan and in the USA some years earlier. Dermatological studies using cyclodextrins have been reported in the literature also (20). The price of cyclodextrins was mainly responsible for the relatively low number of practical applications in different areas. Fortunately, during the last few years prices have decreased and, therefore, further and new applications of cyclodextrins have become possible. EXAMPLES OF THE USE OF C¾CLODEXTRINS The number of patent applications dealing with cyclodextrins in cosmetic and pharma- ceutical applications is enormous. It is not the aim of this article to cite all of them. According to the above-cited advantages for the use of cyclodextrins, some examples will be given. There will be no special differentiation among the cyclodextrin derivatives used in cosmetic formulations. Due to the extremely large number of commercially available products, this information will be far from being complete. Only as examples will some trade names of cyclodextrin-containing cosmetic products be given. PROTECTION OF THE GUEST MOLECULES Protection against light and oxidation. Tea tree oil shows antimicrobial properties. The pure oil is stable for a month in the absence of light and oxygen. Otherwise the terpenes present in the oil react with light and oxygen in the formation of the skin irritant p-cymene. As a cyclodextrin complex, tea tree oil is stable against light and oxygen and therefore the formation of undesired compounds is prevented. The antimicrobial and antiflammatory properties of tea tree oil are not affected by the complex formation (21). Some commercial products are Epicutin-TT © (Chem. Laboratorium Dr. Kurt Richter GmbH) and Pickelex © (Regena Ney Cosmetic). Hydroquinone is used for skin whitening. In aqueous formulations hydroquinone is stable only in a limited-pH range. Therefore stabilizers are normally used. Due to the prevention of the oxidation of hydroquinone, the cyclodextrin complexes have a greater stability. They also show a greater alepigmentation than the hydroquinone itself (22). Kojic acid is another substance used as a whitening agent in cosmetic creams. Its use is
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)