176 JOURNAL OF COSMETIC SCIENCE tumors (11). Some kinds of jewelry radiate far-infrared rays. Representatives employed in this study are jade and tourmaline. Jade radiates far-infrared rays, and tourmaline has pyroelectric and piezoelectric properties and radiates far-infrared rays (12). Far-infrared rays radiated from these jewelry powders were estimated, and skin temperature elevation induced by these jewelry powders was estimated by infrared ray thermal analyzer, thermography. MATERIALS AND METHODS MATERIALS Jade powder (NEPHRITE ©, Jungdo Chem. Co., Korea) and tourmaline powder (Tour- maline ©, Adam Kozan Co., Japan) were used for the experiments. The jade was mined and powdered in Korea, and the tourmaline was mined in Brazil and powdered in Japan. All jewelry was powdered in Korea and Japan. The mean radius of each of the powders was 10 micrometers. Cetostearyl alcohol (Cognis Co., USA), glyceryl stearate and PEG- 100 stearate (Arlacel 165 ©, Uniquema Americas, USA), sorbitan stearate (Arlacel 60 ©, Uniquema Americas), polysorbate 60 (Tween 60 ©, Uniquema Americas), cetyl 2-eth- ylhexanoate (Nikkol CIO ©, Nikko Chemicals Co., Japan), liquid paraffin (Matsumura Chemical, Japan), meadowfoam seed oil (Crodapure MDF ©, Croda Inc., USA), glycerine (Glycerine USP ©, Lita Co., USA), and polyacrylamide-C13-14 isoparaffin-laureth- 7(0.3:0.6:0.1) (Sepigel 305 ©, SEPPIC, France) were used for the O/W emulsion. All water was purified with a reverse osmosis membrane filter. Formulario,. An O/W cream type emulsion was prepared with the jade powder and the tourmaline powder. The formulation example is shown in Table I. J-cream contained 1% Table I. Exemplified Formulation for the O/W Emulsion Concentration (w/w %) Compounds Control J-Cream T-Cream Internal oil phase Cetostearyl alcohol 2.00 2.00 2.00 Arlacel 165 0.80 0.80 0.80 Arlacel 60 0.40 0.40 0.40 Tween 60 1.20 1.20 1.20 C.E.H. 5.00 5.00 5.00 Liquid paraffin 4.00 4.00 4.00 Crodapure MDF 6.00 6.00 6.00 Aqueous phase Glycerine 5.00 5.00 5.00 Sepigel 105 1.00 1.00 1.00 Nephrite -- 1.00 Tourmaline -- -- 1.00 D.I. water To 100 To 100 To 100
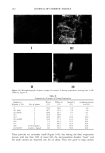
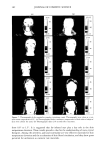
JEWELRY POWDERS AND FAR-INFRARED RAYS 177 jade powder, T-cream contained 1% tourmaline powder, and the control cream con- tained water and oils only. SCANNING ELECTRON MICROSCOPY (SEM) The powders underwent a gold-palladium coating. The observations were realized with a Hitachi S4300 (Hitachi, Japan) scanning electron microscope. FAR-INFRARED RAY ANALYSIS Sample preparation. Each of the jewelry powders was formulated in a cosmetic O/W emulsion. The contents of the jewelry powder were from 0.1% to 7% (w/w). After formulation, they were completely dried in a vacuum oven for 48 hours at 60 ø. Far-infrared ray analysis. Emissivity and emission energies were observed with an IR spectrometer (M2410-C, Midac Co., USA), and the observed wave ranges were 4 to 20 micrometers. THERMOGAPHIC SKIN TEMPERATURE ANALYSIS Preparation of O/W emulsiom. The control cream was formulated with oil, surfactant, and water only, and contained no mineral compounds. Each of the sample creams was formulated with the jade powder and the tourmaline powder. The J-cream was the sample cream containing the jade powder, and the T-cream was that containing the tourmaline powder. The contents of the jewelry powder were 0.25%, 1.0%, 3.0%, and 7.0% (w/w). Subjects and thermography. The subjects were five healthy females and five healthy males aged 26 to 35 years. Each subject washed his or her face using a liquid face-washing agent (Pacific Co.) and water. The subjects were thermographed (Thermovision 900, AGEMA, Sweden) in a room with a constant temperature of 25 ø and a constant relative humidity of 40% to 50%. Ten minutes after washing his or her face, each subject was seated on a chair. Five minutes after seating, the first thermograph was taken. Each subject treated his or her face with 0.5 g of control cream (right side) and 0.5 g of sample cream (left side). Immediately after application of the creams, the second thermograph was taken. The thermographs were all taken according to a pre-fixed schedule. RESULTS AND DISCUSSION The chemical composition of the tourmaline was (Na,Ca)(Mg,Li,A1,Fe2+)3AI6B3Si 6 (OH)4. It had a fibrous structure and a hardness of 7 to 7.5, a specific gravity of 3.0 to 3.3, and a refractive index of 1.625 to 1.655. Tourmaline usually occurs as heavily striated, elongated, prismatic crystals, and less commonly as short, stubby, prismatic crystals. All tourmaline crystals have a rounded, triangular cross section. Tourmaline seldom occurs in tabular crystals, but it also occurs in columnar, radiating, and stalactitic forms, as well as in dense groups of tiny, elongated needles and in black, compact masses.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)