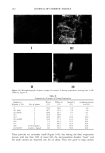
j. Cosmet. sci., 53, 165-173 (May/June 2002) Stability estimation of emulsions of isopropyl myristate in mixtures of water and glycerol C. A. AYANNIDES and G. KTISTIS, Department of Pharmaceutical Technology, School of Pharmacy, Aristotle University of Thessaloniki, Thessaloniki 54006, Greece. Accepted for publication January 31, 2002. Synopsis Phase studies were carried out on systems consisting of isopropyl myristate, polysorbate 80, glycerol, and water. The stable oil-in-water emulsion regions were identified. An influence of the glycerol-to-water ratio on the area of existence of stable emulsions was obtained. The Coulter counter technique was used to determine the droplet size in oil-in-water emulsions. A decrease in average particle size with an increase in glycerol and polysorbate concentration was observed 24 hours after the preparation. Rheologically, the emulsions displayed NewtonJan behavior. Their viscosities increased with increasing glycerol and polysor- bate concentrations. The influence of glycerol and polysorbate concentrations on the cream separation of one-month-old emulsions indicated an increase in emulsion stability with the increase in glycerol and polysorbate concentrations. The use of a polysorbate 80 concentration of 5% by weight can be proposed for stable oil-in-water emulsions of isopropyl myristate in glycerol-and-water mixtures. INTRODUCTION There has been renewed interest in emulsions as a vehicle for delivering pharmaceutical and cosmetic substances, since they have been found to have several advantageous characteristics, including the enhancement of the bioavailability of the drug substance. Oil-in-water emulsions are a convenient form for the administration of lipophilic sub- stances (1,2). This form has several advantages but also the disadvantage of physical instability, which is of great importance for pharmaceutical and cosmetic technology (3,4). The formulation of the most possibly stable emulsions is, therefore, a field of study in pharmaceutical and cosmetic technology (5). In the cosmetic field, emulsification is one of the most useful tools. It is the process of dispersing one material throughout another in separate droplets and, for industry's purposes, effecting a dispersion that will retain its physical characteristics for a reason- able time. Non-ionic surfactants are widely used in cosmetic emulsions because of their lack of toxicity and low sensitivity to additives. These non-ionic surfactants adsorb onto Address all correspondence to G. Ktistis. 165
166 JOURNAL OF COSMETIC SCIENCE the emulsion droplets and maintain stability by creating a hydrated layer on the hy- drophobic particles in oil-in-water emulsions. The approach of particles with hydrated macromolecules adsorbed to their surface leads to repulsion because of a steric interaction of the adsorbed stabilizing chains. In a stable emulsion the emulsifier must cover completely the surface of the droplets of the dispersed phase. If the concentration of emulsifier is lower than the prerequisite to cover the total surface of the droplets, they coalesce to form bigger droplets until their total surface is completely covered by the emulsifier. There is a minimum emulsifier concentration, under which the emulsion is unstable, because the droplet size, formed by the coalescence, is very large. An increase in the emulsifier concentration offers the chance for the formulation of a stable emulsion with smaller droplets. However, after a certain point, the increase in emulsifier does not affect droplet size, and therefore the excess of emulsifier remains in the continuous phase of the emulsion. The increase in emulsifier sometimes leads to the opposite result, namely increases in the average droplet size of the internal phase of the emulsion (6). The addition of a viscous liquid, like glycerol, in the external phase of an oil-in-water emulsion, could improve the stability of the emulsion. As the concentration of the viscous liquid is increased, the viscosity of the external phase is increased and the movement of droplets is decreased, thus delaying the creaming and coalescence. The effect of methylcellulose, as an auxiliary emulsifier, to enhance the stability of mineral oil-in-water emulsions stabilized with nonionic emulsifiers was investigated (7). The results suggest that methylcellulose would associate with polyoxyethylene type nonionic emulsifiers. In the present work, oil-in-water emulsions were prepared with isopropyl myristate as internal phase, polysorbate 80 as emulsifier, and solutions of glycerol in water as external phase. The stability of emulsions was estimated from the average droplet size of the internal phase, assessed by a Coulter counter (8,9). The influence of the concentrations of emulsifier and glycerol on the stability of emulsions was examined. The optimum values of all those concentrations were determined in such a way, so as to prepare stable emulsions of isopropyl myristate in glycerol and water mixtures. EXPERIMENTAL MATERIALS Polysorbate 80 (polyoxyethylene 20 sorbitan monooleate, Tween© 80), isopropyl my- ristate (98% pure), and glycerol 99% were supplied by Sigma Chemical Co. (Saint Louis, MO) and used as received. In every case, water was distilled from an all-glass apparatus. DETERMINATION OF EMULSION AREAS The boundaries of the oil-in-water emulsion domains in isothermal ternary phase dia- grams were determined by progressive titration. A series of mixtures of glycerol and water, with different mass ratios, was chosen. At each value of the ratio, the solution of glycerol in water was progressively titrated in a mixture of polysorbate and isopropyl myristate characterized by a selected value of the isopropyl myristate to polysorbate mass ratio. The concentrations of the solution of glycerol in water, where an oil-in-water emulsion was formed, were derived from weight measurements. By repeating this experiment for
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)