PHASE BEHAVIOR OF ot-HYDROXYOCTANOIC ACID 157 Laureth 4 Water B A 2 White oil 2-hydroxyoctanoic acid Figure 5. Three-dimensional phase diagram of the total system. O: sample A. I: sample B. S: solid area. L2: solubility area. LLC: lameIlar liquid crystal. Region 1: isotropic solution with oil/surfactant at 90/10 wt. ratio. Region 2: isotropic solution with oil/surfactant at 80/20 wt. ratio. Region 3: isotropic solution with oil/surfactant at 70/30 wt. ratio. The phase behavior of the water/white oil/Laureth 4 system (Figure 4) shows one isotropic region (L2) , where white oil and Laureth 4 are totally miscible in each other, with a maximum solubility of water of 15 wt%. The liquid crystal region solubilized up to 7.8 wt% of white oil. There are two three-phase regions: one region connects white oil to LLC and L 2 (L 2 + LLC + white oil), and the second region connects water, white oil, and the LLC phase (LLC + H20 + white oil). There are three two-phase regions in this diagram: (H20 + LLC), (LLC + white oil), and (LLC + L2). The tie lines of two-phase regions are shown in Figure 4 connecting LLC and L 2. Figure 5 shows essential features of the four components ot-hydroxyoctanoic acid/water/ white oil/Laureth 4. The interlayer spacing results from the Laureth 4 with water lameliar liquid crystal are given in Figure 6. Increasing the ratio of Laureth 4/ot- hydroxyoctanoic acid leads to an increase in the slope of the linear relation between the interlayer spacing and the volume ratio of water, but a decrease in the interlayer spacing when extrapolated to zero water content. Figure 7 gives the interlayer spacing upon adding the white oil. Adding 5% white oil does not effect the d o value, but the slope
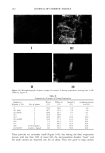
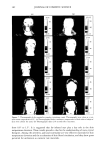
158 JOURNAL OF COSMETIC SCIENCE 75 70 65 60 'D 50 ,- 45 '• 40 35 30 25 20 0.0 ' I ' I ' I ' I 0.2 0.4 0.6 0.8 1.0 volume of water/volume of (Laureth 4+ 2-hydroxyoctanoic acid) Figure 6. Interlayer spacing versus volume ratio of water/(Laureth 4 + o•-hydroxyoctanoic acid). Alpha- hydroxyoctanoic acid/Laureth 4: ß 0/100, ß 5/95, /• 8/92, and x 10/90. increases from 40.03 • to 44.02 •. However, adding 10% changes d o from 32 • to 38.7 • with no change in the slope, as shown in Table I. The liquid crystal phase is a lameliar liquid crystal, LLC, consisting of amphiphilic bilayers separated by water layers, as shown by its optical microscope pattern (Figure 8). EMULSIONS The first sample was a multiphase emulsion, sample A, with a composition of water (70%), white oil (20%), o•-hydroxyoctanoic acid (5%), and Laureth 4 (5%). When the sample was centrifuged at low speed, three layers were observed (Figure 9). These are from bottom water (water, Figure 5), oil (white oil, Figure 5), and microemulsion (L2, Figure 5). The aqueous layer contains liquid crystals (LLC, Figure 5), and the micro- emulsion layer contains crystals of the acid (S•, Figure 5) with dissolved water, oil, and surfactant. Water evaporation showed changes in the layers and their contents (Figure 9) and in the emulsions (Figure 10). Table II gives the composition of sample A during evaporation. Figure 10 shows the emulsion to be water continuous with two kinds of droplets: oil droplets and microemulsion droplets. The dark droplets (Figure 10) are the microemulsion droplets. They are dark because of the o•-hydroxyoctanoic acid crystals inside them.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)