Cosmet. Sci., 53, 185-191 (May/June 2002) Applications of cyclodextrins in cosmetic products: A review HANS-JORGEN BUSCHMANN and ECKHARD SCHOLLMEYER, Deutsches Textilforschungszentrum Nord-West e. V., Adlerstrasse 1, D-47798 KreJ•ld, Germany. Accepted for publication January 31, 2002. Synopsis Cyclodextrins are non-toxic cyclic polysaccharides. They form inlusion complexes with numerous organic molecules. The physical and chemical properties of the guest molecules change due to complex formation. Thus, for example, the stability of the complexed molecule against light and oxygen increases and the vapor pressure is reduced. The solubility of slightly soluble molecules increases in a cyclodextrin complex. All these and further advantages of cyclodextrins and their complexes can be used for the formulation of cosmetic products. As a result, effects are possible not realizable with common techniques. INTRODUCTION The first reference to cyclodextrins was published in 1891 (1). Some years later Schar- dinger also observed the formation of cyclodextrins (2). At this time nothing was known about the structure of these molecules. Freudenberg continued in studying these com- pounds obtained from starch (3). He called them Schardinger dextrins. Further studies by him and Botcherr showed the cyclic structure of Schardinger dextrins (Figure 1) (4,5). Beginning at that time, they were also called "cyclodextrins." Cramer realized that these cyclodextrins were able to include neutral molecules within their cavities (6). From that time on, the interest in cyclodextrins increased. However, cyclodextrins were avail- able only in small quantities. Thus no practical applications seemed to be suitable for these molecules. However, in 1980 Saenger published a review article about cyclodex- trins (7) in which he mentioned some industrial applications. The First International Cyclodextrin Symposium organized by Szejtli took place in Budapest in 1981 (8). One year later the first cyclodextrin book written by Szejtli was published (9). Since then an increasing interest in cyclodextrins and their possible applications has existed. Due to improvements in the production of cyclodextrins, their prices have dropped significantly during the last twenty years. As a result, more industrial applications have become possible and the increasing demand has caused a further reduction in production costs for cyclodextrins. Up to now mainly pharmaceutical applications have been de- scribed in the literature (10,11). Some further applications in analytical chemistry, food, 185
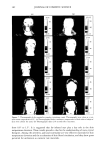
186 JOURNAL OF COSMETIC SCIENCE •' rOH HO'¾ HO b-X 'O•oo • "OH OH Figure 1. Structure of cyclodextrins (dimensions in pro). and very recently textiles are known (12-14). Only a few applications of cyclodextrins in cosmetics have been published (12,13,15-17). Therefore, it seems to be worthwhile to discuss in general possible applications of cyclodextrins in cosmetic products and to give some examples of their present uses. PROPERTIES OF CYCLODEXTRINS AND THEIR COMPLEXES Cyclodextrins are formed during the enzymatic degradation of starch. They are poly- saccharides built from six to eight (o• = 6, ]3 = 7, •/= 8) D-glucose units. The D-glucose units are covalently linked at the carbon atoms C• and C 4. The radii of the rigid cavities vary from 0.50 to 0.85 nm. In these cavities guest molecules can be enclosed. For the complex formation between cyclodextrins and guest molecules, agreement between the size of the cavity and of the guest molecule is not essential. The complex formation takes place only if a part of the guest molecule is located inside the cavity. Due to the complex formation, the physical and chemical properties of the enclosed guest molecules change, e.g., the vapor pressure is reduced and the solubility in water increases. The most important advantages for the use of cyclodextrins in cosmetics are: (1) Protection of the guest molecules against: © decomposition reactions induced by light or heat © oxidation or hydrolysis © chemical reactions with other organic compounds © loss by evaporation (2) Solubilization of the guest molecules in water: © increase of solubility © increase of the rate of solubilization © avoidance of organic solvents © change of viscosity
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)