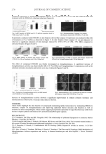
JOURNAL OF COSMETIC SCIENCE 236 A likely hypothesis for the above striking anti-humidity observation is that we think the water uptake ability of trehalose is in its different solid-state forms. While trehalose is nor- mally found in its dihydrate crystalline form, it can under certain conditions exist in an amorphous or glassy form, and these forms can interconvert depending on temperature and humidity (11–13). Here we have investigated the sorption isotherms of two forms of trehalose, one of which is the crystalline dihydrate and the other a “part-crystalline/part-glassy” trehalose form (Figure 4). Concurrently, the dihydrate and the part glassy samples were studied using powder x-ray methods (Figure 5). From the powder x-ray graphs in Figure 5, it is clear that the one sample is far more crystalline than the other. In the literature (14), the water uptake profi le of trehalose glass shows that the glassy form picks up water up to ~12% of its weight until ~50% RH (this is approximately 60% more than the water uptake shown here in Figure 4 for the part-crystalline/part-glassy mate- rial, suggesting that this sample may contain 40% crystalline material). Further increase in %RH results in a slight drop in the water uptake, and then a steady state is reached corre- sponding to a stable form. The fi nal fi gure suggests that the part-crystalline/part-glassy material has fully converted to the dihydrate crystal, which as Figure 4 (dashed line) shows, does not pick up any water. Irrespective of the actual amount picked up by the part-glassy material, the important fi nding here is that water is taken up in this material until about 50% RH and a further increase in %RH results in a slight drop in moisture uptake initially, with no further moisture gain. Figure 3. Adsorption isotherms at ~25°C of 2% trehalose-treated (and ironed) hair (dashed line), and control and ironed hair (dotted line) obtained using a DVS kit (within two days of the heat-styling treatment without any rinse or washing in between), showing very small differences in water uptake, with the trehalose-treated switches showing slightly higher moisture gain. Also given is the normal DVS curve (solid line) for untreated and un-ironed virgin hair. It is noticeable that the effect of heat treatment (200°C irons) has changed the shape of the DVS curves.
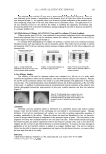
TREHALOSE IN HAIR CARE 237 Here it is hypothesized that the straightening process of a hair switch soaked in trehalose solu- tion produces in situ trehalose glasses on or in the hair fi ber during the heat drying process. These glasses then scavenge water at high humidity—water that may have otherwise dis- turbed the “water wave” (15,16) leading to loss of straight hair style. That trehalose glasses act as water sinks and convert to dihydrate crystals is well documented here the trehalose glasses would pick up about 11–12% of their own weight in water and “lock” them in their di-hydrate crystal form (14). This could explain the fi nding that both sets of switches take up the same amount of water—it is just that in one case some of the water (albeit a very small proportion) is locked in trehalose di-hydrate crystal and not available to disturb the styling water wave. Recent evidence (17,18) suggests that trehalose glasses, depending on their starting point, might pick up nearly four waters per molecule just prior to crystallization. Thus if trehalose is in a glassy state below 50% RH, it can take up water, and as the humidity goes above 50% any glass present converts to a stable dihydrate crystal after which there would be no further pickup of water. What this implies is that the relative humidity conditions during the straightening style creation process may be important for the anti-humidity effect observed. Figure 4. Water uptake DVS curves of trehalose dihydrate (dashed line) and trehalose part-crystalline/part- glassy material (solid line). The dihydrate form shows no water uptake until 80% RH. However, the part-glassy form shows moisture uptake until 50% RH and no further uptake after 60% RH. The “corrected” values on the right hand y-axis assume approximately 40% crystalline material in the part-crystalline/part-glassy sample. Figure 5. Powder x-ray patterns of trehalose dehydrate crystal (dashed line) and part-crystalline/part-glassy material (solid line).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)