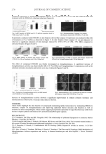
JOURNAL OF COSMETIC SCIENCE 258 seconds, required to produce mild but defi nite erythema with clearly defi ned borders for the unprotected test site. More simply, MED protected site SPF MED unprotected site RESULTS The panel of subjects had 16 males (80%) and 4 females (20%), and had 13 Fitzpatrick skin phototype II (65%) and 7 Fitzpatrick skin phototype III (35%) subjects. There were no Fitzpatrick skin phototype I subjects. The subjects’ mean age was 36 years (minimum = 18 years maximum = 57 years). The SPF values obtained in this clinical trial are shown in Table I. The control standard exhibited an SPF of 16.5 (StDev = 1.62), which is consistent with the expected SPF of 16.3. The test site that had been treated with the control standard followed by sand #1961 exhibited an SPF of 18.3 (StDev = 2.2). The test site that had been treated with the con- trol standard followed by sand #1962 exhibited an SPF of 18.4 (StDev = 2.0). The test site that had been treated with the control standard followed by sand #1152 exhibited an SPF of 17.5 (StDev = 2.2). Thus, the SPF for each test site treated with the control standard followed by sand was essentially the same as the test site treated only with the control standard. There is no signifi cant difference in the average SPF with or without sand. CONCLUSIONS Under the conditions of this clinical trial, there is no signifi cant difference in the average SPF with or without sand. However, the fi ne sand was diffi cult to remove and the coarse sand was unpleasant to the subjects. Thus, our data suggests that Quickrete® commercial sand grade #1962 is preferred for sand-resistance testing. The next step should be the assessment of sand resistance in commonly used sunscreens, especially those with water-resistance claims. However, the Final Rule (3) does not allow for a sand-resistance SPF related claim, which suggests that additional research might fi rst require regulatory review. REFERENCES (1) Sunscreen drug products for over-the-counter human use, Final Monograph, Federal Register, 64, 27666–27693 (1999). (2) D. S. Berger, Specifi cation and design of solar ultraviolet simulators, J. Invest. Dermatol., 53, 192–199 (1969). (3) Labeling and effectiveness testing sunscreen drug products for over-the-counter human use, Federal Register, 76, 35620–35665 (2011).
J. Cosmet. Sci., 63, 259–265 ( July/August 2012) 259 Inhibition of sebum production and Propionibacterium acnes lipase activity by fullerenol, a novel polyhydroxylated fullerene: Potential as a therapeutic reagent for acne SHIGEKI INUI, HISAE AOSHIMA, MASAYUKI ITO, KEN KOKUBO, and SATOSHI ITAMI, Department of Regenerative Dermatology, Graduate School of Medicine, Osaka University, 2-2, G2 Yamadaoka, Suita, Osaka, 565-0871 Japan (S. In., S. It.) Vitamin C60 BioResearch Corporation, 1-3-19 9F Yaesu Chuo-ku, Tokyo, 103-0028 Japan (H.A., M.I.) and Division of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan (K.K.). Accepted for publication December 27, 2011. Synopsis Oxidative stress plays a major role in acne formation this suggests that oxygen-radical scavengers could be potential therapeutic agents. Fullerenol C60(OH)44, a recently developed polyhydroxylated fullerene, is a spherical carbon molecule that has many hydroxyl groups capable of potent radical-scavenging activity. We have investigated its inhibitory effects in vitro on sebum production in hamster sebocytes and in Propionibacterium acnes lipase activity. Sebum production was signifi cantly reduced by 1.5 μM of fullerenol in cells that had been irradiated with 10 mJ/cm2 UVB, although it was not altered in the non-irradiated cells, indicating that fullerene is a sebum suppressor for sebocytes under oxidative stress, such as that induced by UVB. It was also found that fullerenol has inhibitory activity against P. acnes lipase. These results suggest that fullerenol could be a beneficial skin care reagent for control- ling acne vulgaris by suppressing sebum in the infl ammatory response and by reducing P. acnes lipase activity. INTRODUCTION Acne vulgaris is a common infl ammatory skin disease of the pilosebaceous follicles it is characterized by a multifactorial pathogenesis that includes sebaceous gland hyperplasia, increased sebum production, hyperkeratosis of hair follicle pores, and Propionibacterium acnes colonization. However, previous studies have shown the major roles of oxidative stress in the pathophysiology of acne (1–6). Superoxide dismutase (SOD) activity is lower, and hydrogen peroxide generation increased (4) from the Address all correspondence to Shigeki Inui. Address all e-mail to inui@r-derma.med.osaka-u.ac.jpo
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)