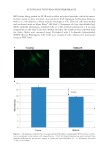
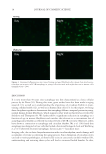
JOURNAL OF COSMETIC SCIENCE 2 the hair are colored and large enough so that they do not easily leach out from the hair structure. They also have affi nity to hair, presumably due to van der Waals or other nonco- valent interactions between the dye and the internal structural components of the hair, al- lowing them to remain in the hair structure even during shampooing or rinse out (3). Hair dye deposition and water fastness is infl uenced by the permeability of primary inter- mediates into the hair and the health condition of the ultrafi ne structural components of hair. Typically, oxidative hair dyeing is carried out at high pH (~10), utilizing ammonia or ethanolamine, causing cuticle swelling and facilitating entry of the dye intermediates into the fi ber structure as well as to decompose hydrogen peroxide so that the dyes can be activated (4). This high pH damages many of the lipids on the surface of the hair, making it a more hydrophilic substrate with greater capacity to absorb ingredients (5). In addi- tion, bleaching, dyeing, and other harsh chemical treatments damage the ultrafi ne struc- ture of hair leading to an overall more porous, or open structure, allowing dye molecules to easily diffuse into and out of the hair (6–8). The ease with which a molecule diffuses into or out of the fi ber is dependent on a number of factors including its molecular size (9). In fact, kinetic studies of dye removal by Wong et al. demonstrated that smaller dyes rinse out much more readily than larger dyes (10). In addition to surface damage, oxidizing agents in permanent hair dye systems also dis- solve melanin and oxidize hair keratin substrate (8). Therefore, both the hair surface bar- rier of the cuticle and the ultrafi ne structure of the cortex are greatly changed. To date, there has not been a comprehensive study on the mechanism of hair dye deposition and leaching pathways published in the literature due to the complexity of hair damage caused by the dyeing process. In this study, we investigated hair damage by consecutive dyeing– shampooing equivalent cycles and elucidated dye deposition and leaching pathways. This information will be helpful for scientists to develop improved technologies that minimize the amount of damage in the coloring process, prevent color fading from washout, and even create specialized products that restore health and brilliance to colored hair. MATERIALS AND METHODS A number of experimental procedures and instruments were used to gain a better under- standing of the dye leaching process and the damage associated with the bleaching and dyeing of pigmented and nonpigmented hair. As already mentioned above, instrumental techniques were employed to investigate the morphological characteristics of the ultrafi ne structure of hair, and consisted of atomic force microscopy (AFM), differential scanning calorimetry (DSC), and dynamic contact angle analysis. In addition, we monitored the deposition of dye within the fi ber structure using refl ected light microscopy and Fourier transform infrared spectroscopy (FT-IR) spectroscopic imaging. Further, studies of the kinetics of dye leaching were carried out by exposing dyed hair to a number of rinse cycles with water while monitoring the aqueous dye concentration. HAIR TRESS PREPARATION The experiments were conducted on both European white and dark brown hair purchased from International Hair Importers (Glendale, NY). Hair tresses were prepared by gluing ~2 g of fi bers together at the hair root to a Plexiglas tab with Duco cement. The resulting dimensions of the hair tresses were 6.0 inches in length by 1.25 inches in width. Hair
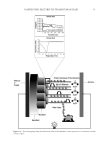
HAIR DAMAGE DURING MULTIPLE OXIDATIVE DYEING AND SHAMPOOING 3 tresses were precleaned with a 3% ammonium lauryl sulfate solution, rinsed thoroughly, and dried prior to use in the experiments. Hair was then subjected to a bleaching regimen for 30 min with Clairol BW2 (Procter & Gamble, Cincinnati, OH) beaching powder and 20-volume hydrogen peroxide (Clairoxide 20 Procter & Gamble). MULTIPLE HAIR DYEING–LEACHING CYCLES In this study, hair was subjected to a regimen that consisted of a dyeing step followed by a dye leaching step, which was carried out by immersing freshly dyed hair fi bers in water for 30 min at 40°C (called one cycle). Five dyeing–leaching cycles were conducted on both white and dark brown hair. During the dyeing steps, 12 hair tresses were dyed with Textures and Tones 4R (Red Hot Red) hair dye (Procter & Gamble, Cincinnati, OH) for 40 min. Hair tresses were then rinsed for 2 min under hot water (~38°C) and excess water was removed by forming a squeegee with the index and middle fi ngers and running them along the length of the tress. Hair tresses were then dried with a hair blow dryer (tem- perature set to medium). During the dye leaching process, each freshly dyed hair tress was suspended in a vessel containing 500 ml of water at 40°C with continuous stirring at 50 rpm. The total dye leaching time is 30 min. Leached dye was measured with an auto- mated dissolution system, consisting of a VK7000 dissolution testing apparatus (Varian Inc., Cary, NC) and an ultraviolet–visible spectrophotometer equipped with seven fl ow cells and a fl ow pump (Agilent Technologies, Santa Clara, CA). The dye concentration in solution was determined by measuring the absorbance at 490 nm. T he amount of the dye leaching from hair was calculated from the ratio of absorbance to the weight of the hair tress. The continuous dye release from hair fi bers within 30 min was measured at differ- ent times with a 2-min interval. Six repetitions per sample were measured. Hair dye leaching cycles were performed by rinsing with water. Surfactant was not added as it in- teracts with hair dye and interferes with dye detection. FT-IR SPECTROSCOPIC IMAGING A 1-cm-long hair bundle was cut from the middle of the hair tresses and mounted on the top of a sample holder by embedding in ice. The hair bundle was then microtomed at -30°C into 5-μm-thick cross sections with a Leica CM 1850 cryostat (Leica Microsystems Inc., Bannockburn, IL). Hair cross sections were collected onto CaF2 windows for IR imaging. This preparation technique avoids any possibility of contamination with embedding or fi xing medium. Hair cross sections were imaged with a PerkinElmer Spotlight system (PerkinElmer, Inc., Waltham, MA) that couples a FT-IR spectrometer with an optical microscope. The system consists of a linear array mercury cadmium telluride detector and an automated high precision x-y sample stage. Images were acquired with a 6.25 μm step size, eight scans for each spectrum, and 8 cm-1 spectral resolution. IR spectra were analyzed using ISys 5.0 software (Malvern Instruments, Inc., Malvern, Worcestershire, UK). ATOMIC FORCE MICROSCOPY The hair cross sections were prepared by the following procedure. A single hair fi ber was hung in the center of a cylinder. Buehlers Epoxicure™ Resin (Buehler, Lake Bluff, IL) and Buehlers Epoxicure™ Hardener (Buehler) were mixed (weight ratio of 5:1) and slowly poured into a cylinder. To ensure the hair fi ber remained vertically positioned in epoxy, one end of the hair fi ber was attached to a thin pole, and the other end was tied with an
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)