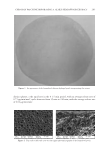
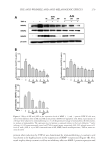
JOURNAL OF COSMETIC SCIENCE 254 (M, W) or 72 h (F), ensuring continuous exposure to the test material over 4 weeks. A positive and negative control must be included because of the wide variation in responses (7). A follicular biopsy is taken of the test sites. Cyanoacrylate glue is applied to a glass slide, which is then turned upside down and placed fi rmly on the skin at the test site, so that the glue is in contact with the test site. Upon drying, the slide is rapidly ripped off the skin to maintain the integrity of the biopsy specimen. The slide is then examined micro- scopically for the number of follicles and microcomedones per area. Some calculate the ratio of follicles to microcomedones to calculate the percent microcomedone formation. BENEFITS For more than 30 years, this human model of comedogenesis has been used as a tool by dermatologists to evaluate the ability of a cosmetic ingredient/product to induce comedo- nes. Follicular biopsy is a better model system than the rabbit model. Unlike the rabbit model, the follicular biopsy model is human based, increasing the expected relevance of the generated data. The follicular biopsy method seems to be more predictive than the rabbit model. Although the follicular biopsy model system is human based, the model system is relatively inexpensive. In conclusion, the follicular biopsy model system is a better model than the rabbit system, human based, and relatively inexpensive. RISKS Despite more than 30 years of use, the follicular biopsy model system has never been vali- dated against an in-use clinical trial, the gold standard. Thus, the relevance of the data generated by the follicular biopsy method should not be used to claim non-comedogenecity of an ingredient or fi nished product. Furthermore, the data generated by the follicular biopsy method should not be used to claim comedogenicity of a fi nished product, because the model system does not predict that fi nished products containing comedogenic ingredients are comedogenic (7). One reason for the questionable relevance of the follicular biopsy model is that most cos- metic products are used on the face or facial area, not on the back. The relationship of comedone/acne formation on the back to formation on the face has never been established. Although follicular biopsy has been performed on the face, this practice is not encouraged because of the potential for scarring when the glass slide is rapidly ripped from the skin. This is also a concern on the back biopsies, but of less concern to most subjects. In conclusion, the follicular biopsy model system has never been validated against the gold standard, comedones developed on the back have never been validated as a model for the face, the test material is used in a manner not intended, and the follicular biopsy model can induce scarring on subjects. IN-USE CLINICAL TRIAL METHOD Individuals with comedones and/or acne are recruited into the clinical trial. Subjects must have Grade I (mild), Grade II (moderate), or Grade III (severe) lesion categories.
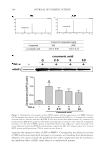
NON-COMEDOGENIC AND NON-ACNEGENIC CLAIM SUBSTANTIATION 255 Subjects must have not more than 25 facial lesions, which are predominately open and closed comedones. A board-certifi ed dermatologist evaluates the lesions on the face and the results are recorded. Typically, the subject uses the test material on the face for 4–6 weeks. For a 4-week trial, evaluations occur after 2 weeks (optional) and 4 weeks of test material use, whereas for a 6-week trial, evaluations occur after 3 weeks (optional) and 6 weeks of test material use. A board-certifi ed dermatologist evaluates the lesions on the face and the results are recorded. Differences between baseline and interim or fi nal evaluations are considered statistically signifi cant if the probability of obtaining the results by chance is 0.050 using analysis of variance and/or t-test statistical analysis. BENEFITS The in-use clinical trial is a better clinical test than the follicular biopsy and a more pre- dictive test than the follicular biopsy. Like the follicular biopsy model, the in-use clinical trial is human based. However, the in-use clinical trial is the gold standard for comedogenicity because it uses the test material in the manner expected and, it uses the skin of the face, which is more susceptible to comedones and acne. In this regard, the in-use clinical trial is similar to acne trials used to support approval of acne medications by the Food and Drug Administration. A positive control, a material known to cause comedones, and a negative control, a material known not to cause comedones, are not needed because each subject at baseline serves as their own control. Each subject can generate comedones because they must have comedones to qualify for the trial. Thus, each subject enters the trial with the capacity to generate comedones. Consequently, a positive control is not necessary. Indeed, one could argue that including a positive control would be in confl ict with the principles of Good Clinical Practice as described in the World Medical Association’s Declaration of Helsinki (as amended). Other examples of a clinical trial in which a positive control is unethical and inhuman are allergy trials, such as photoallergy. In conclusion, the in-use clinical trial is a better model than the follicular biopsy trial, based on facial skin instead of back skin, and based on the use of the test material in a manner intended. The in-use clinical trial is the gold standard. RISKS The primary risk of the in-use clinical trial is the expense. Conducting an in-use clinical trial is more expensive than the follicular biopsy model, which is why some companies do not conduct in-use clinical trials. CONCLUSIONS In conclusion, the in-use clinical trial is the gold standard when determining comedo- genicity of an ingredient or product. The follicular biopsy model has defi ciencies com- pared with the in-use clinical trial, and the rabbit model has defi ciencies compared with the follicular biopsy model. Although more expensive, the in-use clinical trial uses the face,
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)