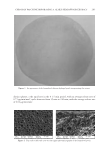
JOURNAL OF COSMETIC SCIENCE 252 (3) K. O. Fa tma, Ö. Selda, and A. Duygu, Microbiological investigation of used cosmetic samples. Hacettepe Univ. J. Faculty Pharm., 30, 1–16 (2010). (4) S. S. Sne ha and K. M. Varsha, Study of bacterial contaminants in local as well as branded lipsticks before and after consumer use. Int. J. Recent Adv. Multidiscip. Res., 2, 149–154 (2015). (5) J. Basil, J. R. Alan, C. R. Annette, and A. Michael, A survey of microbiological contamination in cos- metics and toiletriesin the U.K. (1971). J. Soc. Cosmet Chem., 25, 563–575 (1974). (6) H. J. Muh ammed, Bacterial and fungal contamination in three brands of cosmetics marketed in Iraq. Iraqi J. Pharm. Sci., 20, 38–42 (2011). (7) T. Huber, G. Faulkner, and P. Hugenholtz, Bellerophon: A program to detect chimeric sequences in multiple sequence alignments. Bioinformatics, 20, 2317–2319 (2004). (8) O. S. Kim , Y. J. Cho, K. Lee, S. H. Yoon, M. Kim, and H. Na, Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol., 62, 716–721 (2012). (9) L. Han, J . E. Lei, X. Wang, L. T. Guo, Q. Y. Kang, and L. He. Septicemia caused by Leifsonia aquatica in a healthy patient after retinal reattachment surgery. J. Clin. Microbiol., 51, 3886–3888 (2013). (10) L. Porte , A. Soto, D. Andrighetti, J. Dabanch, S. Braun, and A. Saldivia, Catheter-associated blood- stream infection caused by Leifsonia aquatica in a haemodialysis patient: A case report. J. Med. Microbiol., 61, 868–873 (2012). (11) A. Zinch uk, O. Zubach, A. Zadorozhnyj, Y. Chudina, and V. Bilavka, Characteristics of meningitis due to Methylobacterium mesophilicum: A rare case. Jpn. J. Infect Dis., 68, 343–346 (2015). (12) H. F. Je nkinson, H. C. Lala, and M. G. Shepherd, Coaggregation of Streptococcus sanguis and other strep- tococci with Candida albicans. Infect Immun., 58, 1429–1436 (1990). (13) N. Norsk ov-Lauritsen, Classifi cation, identifi cation, and clinical signifi cance of Haemophilus and Aggre- gatibacter species with host specifi city for humans. Clin. Microbiol. Rev., 27, 214–240 (2014). (14) F. Devli eghere, A. De Loy-Hendrickx, M. Rademaker, P. Pipelers, A. Crozier, and B De Baets, A new protocol for evaluating the effi cacy of some dispensing systems of a packaging in the microbial protec- tion of water-based preservative-free cosmetic products. Int. J. Cosmet. Sci., 37, 627–635 (2015). (15) T. L. Ha dfi eld, P. McEvoy, Y. Polotsky, V. A. Tzinserling, and A. A. Yakovlev, The pathology of diph- theria. J. Infect. Dis., 181, S116–S120 (2000). (16) L. Plant , V. Asp, L. Lovkvist, J. Sundqvist, and A. B. Jonsson, Epithelial cell responses induced upon adherence of pathogenic Neisseria. Cell Microbiol., 6, 663–670 (2004). (17) M. D. Lu ndov, L. Moesby, C. Zachariae, and J. D. Johansen, Contamination versus preservation of cos- metics: A review on legislation, usage, infections, and contact allergy. Contact Dermatitis., 60, 70–78 (2009). (18) K. Rajgo pal and S. S. Agarwal, Simultaneous estimation of p-hydroxybenzoic acid and its esters in wash off/leave- on cosmetic products by highperformance thin layer chromatography. Int. J. Pharm. Stud. Res., 2, 100–105 (2011).
J. Cosmet. Sci., 68, 253– 256 ( July/August 2017) 253 Non-comedogenic and non-acnegenic claim substantiation CRAIG WEISS and MICHAEL CASWELL Consumer Product Testing Company, Inc., Fairfi eld, NJ 07004 Accepted for publication May 20, 2017. Synopsis There are currently two methods to evaluate comedogenecity. One is the inexpensive human model developed by Mills and Kligman and modifi ed by others. The second is the more costly human clinical trial, which is the gold standard for comedogenesis and to which the human model is compared. The qualifi cation of each method to support the comedogenecity claim is evaluated and contrasted. BACKGROUND “Acne cosmetic” was a term created by Mills and Kligman (1) to describe the development of comedones and/or acne in patients, typically middle-aged females, who would not normally be expected to develop such. The rabbit ear model was quickly developed and large amounts of data were generated using the model (2–5). The rabbit model was not a perfect predictor of comedogenesis in a human model (5,6). In 1989, at an invitational symposium on comedogenicity, the group wrote “If the animal model does not show evidence of comedogenesis, the test material under consideration is unlikely to be comedogenic in human skin (7).” Thus, the experts in 1989 wrote that the rabbit model did not accurately mimic comedogenesis in humans. Whereas the rabbit model is an adequate fi rst screen for comedogenicity, its inherent inability to mimic human comedogenesis has relegated it to a screening tool. There are currently two methods to evaluate comedogenecity. One is the inexpensive human model developed by Mills and Kligman and modifi ed by others (8). The second is the more costly human clinical trial, which is the gold standard for comedogenesis and to which the human model is compared. FOLLICULAR BIOPSY MODEL METHOD Individuals with prominent follicles on the upper back are recruited into the clinical trial. The upper back is patched with approximately 0.2 ml of each test material for 48 h Address all correspondence to Michael Caswell at MCaswell@cptclabs.com.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)