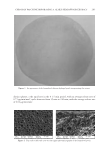
JOURNAL OF COSMETIC SCIENCE 258 This suggests the potential of the extract for application in the depigmentation process. However, the incorporation of this extract in cosmetic formulations such as an oil-in-water emulsion is limited because of its low solubility in an aqueous phase, thus affecting its permeability through skin and its effi cacy at the action site. Artocarpin has poor ability to permeate through skin because of its large molecular weight (MW 400) and lipophi- licity (log partition coeffi cient 4). To improve this limitation, we formulated the extract into a form that can promote skin penetration of the compound, leading to enhanced depigmenting effi cacy. The hydrogel patch is a dosage form that is designed particularly for the delivery of the active compounds into the skin for fast activation of the desired amount which results from hydration of the stratum corneum. The hydrogel patch that we developed required an aqueous polymer to act as a rate-controlling matrix. Chitosan, a natural aqueous poly- mer, attracted our interest because of its biodegradability and nontoxicity. Previous stud- ies had shown that a patch prepared from chitosan exhibits good bioadhesion to the skin because of its net positive charge properties (9,10). In the present study, to incorporate the extract into the chitosan hydrogel patch, the extract was initially formulated into an oil-in-water (o/w) microemulsion, which was then blended with an aqueous solution of chitosan polymer (9). The resulting solution was then cast into a mold to shape the hydrogel patch. We were then able to determine the release charac- teristics of the artocarpin, the major bioactive component of the formulated patch, and test and clinically observe any resulting skin irritation as well as the effi cacy on skin depig- mentation of the formulated patch, and to evaluate its potential for skin depigmentation. MATERIALS AND METHODS PREPARATION OF THE EXTRACT The heartwood of A. altilis was collected on July (raining season) from Phitsanulok Province, Thailand. The heartwood portion was chipped and dried at 50°C by using a hot-air oven. The dried chipped heartwood (1 kg) was then macerated at room temperature for 2 cycles (2 days/cycle) with suffi cient diethyl ether (analytical grade, Labscan Asia, Co., Ltd., Bangkok, Thailand), according to our previous studies (6,7). The extract from each cycle was pooled and fi ltered through a fi lter paper to remove unwanted residue and concen- trated to dryness using a rotary evaporator. The extract was further dried in a desiccator, and kept in a tight amber glass bottle at 4°C for further studies. QUANTIFICATION OF ARTOCARPIN IN THE EXTRACT The content of artocarpin in the extract was determined by using isocratic high performance liquid chromatography (HPLC). The HPLC instrument consisted of an SPD-20A UV detector and an LC-20AP pump (Shimadzu Co., Ltd., Kyoto, Japan). A Phenomenex Gemini C18 column with 5 μm and 250 × 4.60 mm diameter was applied as the stationary phase. The mobile phase consisted of a mixture of methanol (analytical grade, Labscan Asia, Co., Ltd.) : water (80:20). The fl ow rate of mobile phase was set at 1 ml/min, and the injection volume was 20 μl. The quantifi cation of artocarpin was based on the peak area
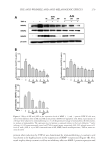
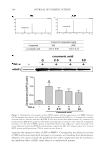
CHITOSAN PATCH INCORPORATING A. ALTILIS HEARTWOOD EXTRACT 259 at 282 nm using a calibration curve of a standard artocarpin. The study was performed in triplicates. The standard artocarpin used in this study was isolated from a diethyl ether extract of A. altilis heartwood, according to previous studies (7,11). FORMULATION OF CHITOSAN HYDROGEL PATCH INCORPORATING THE EXTRACT Preparation of the extract into the oil-in-water (o/w) microemulsion. The o/w microemulsion system consisted of 0.04% w/w extract powder, 1% w/w isopropyl myristate (IPM Nikko Chemicals Pte. Ltd., Jurong Island, Singapore), 12.8% w/w polyoxyethylene sorbitan monosterate (Tween® 80 Nof Corporation, Tokyo, Japan), 6.4% w/w glycerin (Namsiang Trading Co., Ltd., Bangkok, Thailand), and 79.4% w/w of deionized (DI) water. The extract powder was dissolved in IPM to obtain the internal oil phase of the microemulsion sys- tem. The external water phase consisted of Tween® 80, glycerin, and DI water. The water phase was continuously added to the oil phase with slightly mixing. The obtained micro- emulsion was transparent, and the mean hydrodynamic diameter of its internal oil phase was 31.8 ± 1.2 nm as measured thrice by photon correlation spectroscopy employing a Zetasizer (Model ZetaPALS Brookhaven Instruments Coporation, Holtsville, NY). Preparation of chitosan solution. The chitosan used had a molecular weight in the range of 100,000–1,000,000 Dalton and more than 95% degree of deacetylation (Aqua Premier Co., Ltd, Chonburi, Thailand). The total heavy metal and ash contents in chitosan were less than 10 ppm and 2%, respectively. Chitosan with a specifi ed amount at 4% w/w was dispersed in a part of DI water. Then, lactic acid (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) was added to dissolve the chitosan and to control the pH of the chitosan solution in a range of 3–4. The chitosan–lactic acid mixture was stirred at room temperature until a clear yellowish solution was obtained. Formulation of a chitosan hydrogel patch incorporating the extract. The o/w microemulsion containing 0.04% w/w extract was blended with the 4% w/w chitosan solution in a ratio of 1 to 1 by weight. NaCl (Ajax Finechem Pty. Ltd., New South Wales, Australia) at an amount of 1% w/w was then added in the blended mixture. The blended mixture was further agitated for 1 h and left standing until free from air bubbles. The 21 grams of the resultant solution was cast on a clear petri dish with a diameter of 9 cm and kept on a level surface at 35°–40°C in a hot-air oven. After 2–3 d, the casted patch was peeled off from the petri dish and kept for further determinations. The amount of artocarpin in the patch was 0.07 mg/cm2, according to analysis by using HPLC. The thickness of an indi- vidual patch was controlled to 500 ±10 μm. DETERMINATION OF PHYSICOCHEMICAL PROPERTIES OF THE FORMULATED PATCH INCORPORATING THE EXTRACT Mechanical properties. The tensile tester (Instron® Model 4411 S/N H2082 Instron Ltd., Buckinghamshire, UK) was used to determine the tensile strength and percent elonga- tion at break of the formulated patches. The patch specimens were cut out in a rectangle with a length of 70.0 mm and a width of 10.0 mm. The thickness of each specimen was calculated as the average value of three separate measurements taken along the middle of 20 mm. The cross-section area of the tested patch was calculated by multiplying the mean thickness with gauge width. The tested patch was clamped by upper and lower grips. The rate of grip separation was 12.5 mm/min and loading weight was 200 N. The testing
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)