JOURNAL OF COSMETIC SCIENCE 266 Table I Demogr aphic and baseline characteristics of subjects enrolled in the effi cacy study (N = 59) Characteristics Test group (n = 30) Control group (n = 29) p* Age, mean (SD), year 41.3 (6.9) 42.2 (6.9) 0.618 Sex, no. (%) Male 2 (6.7) 1 (3.5) Female 28 (93.3) 28 (96.5) Race, no. (%) Thai 30 (100) 29 (100) Education, no. (%) Primary school 20 (66.7) 18 (62.1) High school 10 (33.3) 8 (27.6) Bachelor’s degree or equivalent - 3 (10.3) Occupation, no (%) Agriculturalist 3 (10) 3 (10.3) Freelance/personal business 5 (16.7) 6 (20.7) Contingent worker 22 (73.3) 20 (69.0) Skin properties, mean (SD) Melanin value, AU 370.8(2.8), 369.1 (4.1) 0.812 Moisture content, AUa 45.7 (3.3) 46.9 (4.3) 0.233 Skin pH 5.6 (0.5) 5.5 (0.5) 0.446 Erythema value, AU 417.7 (3.4) 418.1 (3.4) 0.653 AU: arbitrary unit. *2-Group t-test with a 2-sided signifi cance level of 0.05. a One unit represents a water content of stratum corneum of 0.02 mg/cm2. device for delivering the bioactive compound to the skin. We observed that the bioactive components of the A. altilis heartwood extract were released from the formulated patch at the effective amount, resulting in the depigmenting effi cacy in the clinical study. Our experimental process began with the preparation of the A. altilis heartwood extract. We then analyzed the extract by using HPLC. Our analysis showed a high content of arto- carpin (89.5 ± 6.5% w/w) in the prepared extract, which coincides with the 90.6 ± 5.1% w/w of artocarpin reported in our previous study (7). These results clearly indicate that artocarpin is a major compound of the A. altilis heartwood extract. For the formulation of the chitosan hydrogel patch, lactic acid was used to dissolve chitosan (9,10,13), and the chitosan solution was then blended with the extract microemulsion by stirring. The blended solution did not show incompatibility because of the external phase of the microemulsion being water which is miscible with the aqueous solution of the polymer. The formulated patches showed proper fl exibility with tensile strength in the range of 5–10 N/mm2 and percentage elongation at break point in the range of 20–50%. These values allow the patches to be handled and to adhere to the skin (9,10,14). The fl exibility of the formulated patches is possibly caused by the plasticizer activity of the incorporated ingredients, such as lactic acid (9,10,15), NaCl (16,17), and glycerin (18,19) which together interrupt the compactness of the polymeric network. As well, the hygroscopic behavior of these plasticizers kept the patch in a hydrogel state and main- tained a high moisture content. Water remaining in the hydrogel patch supported the incorporated microemulsion surfactant (Tween® 80) by solubilizing the IPM oil which is a dissolving solvent in the extract.
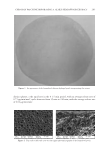
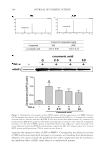
CHITOSAN PATCH INCORPORATING A. ALTILIS HEARTWOOD EXTRACT 267 Table II Measured parameters in the test and control groups. Each value is mean ± SD Parameter Test group (n = 30) Control group (n = 29) Pa % change (test group) % change (control group) Pb Melanin value, AU Week 0 370.8 (2.8) 369.1 (4.1) 0.067 0 0 0 Week 1 367.3 (9.2) 365.3 (3.2) 0.273 2.7 (7.1) 1.5 (4.0) 0.410 Week 3 346.4 (4.4) 364.5 (5.1) 0.001 8.3 (8.9) 1.3 (5.5) 0.001 Week 6 335.8 (8.8) 364.4 (5.9) 0.001 12.5 (12.2) 1.4 (5.2) 0.001 Week 8 317.7 (9.9) 362.8 (2.7) 0.001 19.0 (13.4) 2.3 (4.6) 0.001 Moisture content, AUa Week 0 45.7 (3.3) 46.9 (4.3) 0.233 0 0 0 Week 1 51.3 (1.5) 49.6 (0.9) 0.001 13.2 (18.5) 6.9 (19.4) 0.207 Week 3 51.8 (1.5) 52.3 (1.4) 0.191 20.1 (31.1) 14.7 (28.9) 0.493 Week 6 58.5 (3.1) 53.2 (2.2) 0.001 36.2 (43.8) 16.8 (29.9) 0.053 Week 8 56.4 (2.2) 57.9 (1.7) 0.005 32.2 (45.5) 29.4 (43.6) 0.810 Skin pH Week 0 5.56 (0.04) 5.45 (0.05) 0.001 0 0 0 Week 6 5.45 (0.04) 5.47 (0.04) 0.060 1.7 (4.4) 0.7 (4.7) 0.402 Week 8 5.54 (0.09) 5.32 (0.04) 0.001 0.3 (10.4) 1.7 (10.6) 0.611 Erythema value, AU Week 0 417.7 (3.4) 418.1 (3.4) 0.653 0 0 0 Week 1 421.2 (4.6) 409.3 (4.4) 0.067 1.7 (16.9) 1.0 (11.3) 0.853 Week 3 419.8 (3.3) 408.8 (4.7) 0.059 2.3 (22.4) 1.1 (13.4) 0.805 Week 6 416.3 (3.2) 402.3 (5.1) 0.023 1.4 (17.2) 2.5 (13.8) 0.788 Week 8 414.3 (5.7) 400.4 (4.0) 0.052 0.4 (18.6) 3.7 (12.2) 0.425 Pa: one-way ANOVA compare two mean of each parameter (p 0.05). Pb: one-way ANOVA compare two mean of % change (p 0.05). a One unit represents a water content of stratum corneum of 0.02 mg/cm2. Our previous studies revealed that the surface morphology of the chitosan patch was compact (10,13). However, in the present study, we observed porous structures through- out the formulated patch. It is possible that the presence of other ingredients, such as NaCl in the casting solution interrupted the compactness of the polymeric network of chitosan, resulting in the formation of porosity (17,20). These porous structures infl u- enced the patch fl exibility and the release of the incorporated bioactive components through the patch. We found a high rate and amount of artocarpin release (about 70% of the initial content of artocarpin in the casted patch) in the fi rst 30 min. As the formulated patch was composed of high porous structures, the receptor medium was able to quickly diffuse into the patch. This led to the fast release rate of the artocarpin to the receptor cell (9,21), after which a low amount of artocarpin was released through the patch, because of the low amount of artocarpin remaining in the patch. A 4-h human patch test is an alternative method of determining the acute skin irritation potential of a tested substance (22). When the substance being tested was applied to the skin and then covered with cotton bandage material, such as a Webril pad, the stratum corneum was hydrated. This consequently increased the penetration of the substances through the skin and thereby accelerated the incidence of skin redness and irritation. In the present study, irritation on the volunteers’ forearms was not observed after applying the test patch, whereas skin irritation was clearly observed after applying 20% SLS, which was the positive control for identifying substances or preparations that were classifi ed as
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)