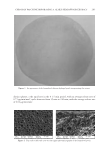
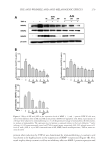
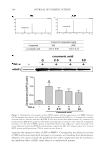
JOURNAL OF COSMETIC SCIENCE 268 irritants (23). When the test patches were applied on subjects’ faces for 8 weeks (three times/week, 30 min for each time), erythema, scaling, and oedema on applied area were not observed, and the skin pH at the applied area was maintained in the normal range (5.5–6.5). These results demonstrate the safe and benefi cial use of the tested patches for use in cosmetics. The outermost layer of the skin is the stratum corneum which plays a role as a barrier layer relying on its lipid and keratin composition and organization. The skin permeation of compounds such as artocarpin, which has a large size (MW 400 dalton) and lipophilic nature (log partition coeffi cient, log P 4) is generally poor (24,25). Reversible alteration of the organization of keratinized protein and/or fl uidization of the lipids by using per- meation enhancers is widely implemented to improve the skin permeation of poor perme- ation compounds. In the present study, the extract enriched with the bioactive compound, artocarpin, was formulated into the hydrogel patch to improve skin permeability of arto- carpin. Water molecules accumulated in the stratum corneum during patch application which contributed to skin moisturization and acted as a permeation enhancer by chang- ing the structure of the keratinized protein in the stratum corneum (26). The microemul- sion nonionic surfactant (Tween® 80) present in the formulated patch also facilitated the permeability of bioactive components by reversely changing the conformation and/or fl uidization of the lipid bilayer (26). We found that the formulated patch containing the extract (0.07 mg of extract/cm2 of patch) signifi cantly improved the hyperpigmented area after 3 weeks of application. This implies that the amount and rate of release of artocarpin from the formulated patch achieved an effective topical delivery, and in addition, a sig- nifi cant increase in the skin moisture content was found after patch application. In conclusion, the artocarpin-enriched A. altilis heartwood extract was formulated into a hydrogel patch by using a microemulaion technique to solubilize the lipophilic extract and by using polymeric chitosan to control the delivery of the artocarpin. The formulated patch was effective in skin depigmentation and was mild on the skin causing no skin red- ness or irritation. However, the small number of subjects in this study did not in- clude the variety of skin types which may be found in studies with a larger more diverse sample. Future studies with a larger number of subjects and a longer duration should be performed to confi rm the benefi cial effects of the formulated patch for improving hyperpigmentation. ACKNOWLEDGEMENTS Financial supports from the Commission on Higher Education, Ministry of Education, Thailand, and International Laboratories Corp. Ltd. We also thank the Center of Excel- lence for Innovation in Chemistry (PERCH-CIC), the S&T Postgraduate Education and Research Development Offi ce (PERDO), the Commission on Higher Education for facil- ity support. Many thanks to Mr. Roy Morien of the Naresuan University Language Cen- tre for his editing assistance and advice on the English expression in this document. REFERENCES (1) M . S. Sikarwar, B. J. Hui, K. Subramaniam, B. D. Valeisamy, L. K. Yean, and K. Balaji, A. review on Artocarpus altilis (Parkinson) Fosberg (breadfruit), J. App. Pharm. Sci., 4, 91–97 (2014).
CHITOSAN PATCH INCORPORATING A. ALTILIS HEARTWOOD EXTRACT 269 (2) K . Shimizu, R. Kondo, K. Sakai, S. H. Lee, and H. Sato, The inhibitory components from Artocarpus incisus on melanin biosynthesis, Planta Med., 64, 408–412 (1998). (3) K . Shimizu, R. Kondo, K. Sakai, N. Takeda, and T. Nagahata, The skin-lightening effects of artocarpin on UVB-induced pigmentation, Planta Med, 68, 79–81 (2002). (4) S . Buranajaree, P. Donsing, R. Jeenapongsa, and J. VIyoch, Depigmenting action of a nanoemulsions containing heartwood extract of Artocarpus incisus on UVB-induced hyperpigmentation in C57BL/6 mice, J. Cosmet. Sci., 62, 1–14 (2011). (5) P . Donsing, N. Limpeanchob, and J. Viyoch, Evaluation of the effect of Thai breadfruit’s heartwood extract on melanogenesis-inhibitory and antioxidation activities, J. Cosmet. Sci., 59, 41–58, (2008). (6) N . Tiraravesit, S. Yakaew, R. Rukchy, W. Luangbudnark, C. Viennet, P. Humbert, and J. Viyoch, Arto- carpus altilis heartwood extract protects skin against UVB in vitro and in vivo, J. Ethnopharmacol., 175, 153–162 (2015). (7) K . Itsarasook, K. Ingkaninan, and J. Viyoch, Artocarpin-enriched extract reverse collagen metabolism in UV-exposed fi broblasts, Biologia, 69, 943–951 (2014). (8) W . C. Lan, C. W. Tzeng, and C. C. Lin, Prenylated fl avonoids from Artocarpus altilis: Antioxidant ac- tivities and inhibitory effects on melanin production, Phytochemistry, 89, 78–88 (2013). (9) P . Boriwanwattanarak, K. Ingkaninan, N. Khorana, and J. Viyoch, Development of hydrogel patch for controlling the release of curcuminoids by using chitosan as controlled-release matrix, Int. J. Cosmet. Sci., 30, 205–218 (2008). (10) J. Viyoch, T. Sudemek, W. Srema, and W. Suwongkrua, Development of hydrogel patch for controlled delivery of alpha-hydroxy acid contained in the extract of tamarind’s fruit pulp, Int. J. Cosmet. Sci., 27, 89–99 (2005). (11) T. Pitaksuteepong, A. Somsiri, and N. Waranuch, Targeted transfollicular delivery of artocarpin extract from Artocarpus incisus by means of microparticles, Eur. J. Pharm. Biopharm., 67, 639–645 (2007). (12) J. Viyoch, Mechanisms of Action of Artocarpus incisus’s Heartwood Extract on Melanogenesis Inhibi- tion, Final Report, Bangkok, Thailand: Thailand Research Fund, Contract No.: DBG5180012 (2009). (13) J. Viyoch, P. Patcharaworakulchai, R. Songmek, V. Pimsan, and S. Wittaya-Areekul, Formulation and development of a patch containing tamarind fruit extract by using the blended chitosan-starch as a rate- controlling matrix, Int. J. Cosmet. Sci., 25, 113–125 (2003). (14) N. Mondal, A. Samanta, T. K. Pal, and S. K. Ghosal, Effect of different formulation variables on some particle characteristics of poly (DL-lactide-co-glycolide) nanoparticles, Yakugaku Zasshi, 128, 595–601 (2008). (15) Q. Meng, M. C. Heuzey, and P. J. Carreau, Hierarchical structure and physicochemical properties of plasticized chitosan, Biomacromolecules, 15, 1216–1224 (2014). (16) B. Zhang, D. A. Hoagland, and Z. Su, Ionic liquids as plasticizers for polyelectrolyte complexes, J. Phys. Chem B, 119, 3603–3607 (2015). (17) L. Moreau, W. Bindzus, and S. Hill, Infl uence of sodium chloride on color development of cereal model systems through changes in glass transition temperature and water retention, Cereal Chem., 86, 232–238 (2009). (18) T. Karbowiak, H. Hervert, L. Léger, D. Champion, F. Debeaufort, A. Voilley, Effect of plasticizers (wa- ter and glycerol) on the diffusion of a small molecule in iota-carrageenan biopolymer fi lms for edible coating application, Biomacromolecules, 7, 2011–2019 (2006). (19) V . Epure, M. Griffon, E. Pollet, and L. Avétous, Structure and properties of glycerol-plasticized chitosan obtained by mechanical kneading, Carbohydr Polym, 83, 947–952 (2011). (20) M. F. Cardinal, M. Lovino, and D. Bernik, Comparative study of the porosity induced by CTAB and Tween as silica templates, Mater Sci Eng C, 27, 75–79 (2007). (21) S. Honary, B. Hoseinzadeh, and P. Shalchian, The effect of polymer molecular weight on citrate cross- linked chitosan fi lms for site-specifi c delivery of a non-polar drug, Trop. J. Pharm. Res., 9, 525–531 (2010). (22) M. K. Robinson, J. P. McFadden, and D. A. Basketter, Validity and ethics of the human 4-h patch test as an alternative method to assess acute skin irritation potential, Contact Dermatitis, 45, 1–12 (2001). (23) J. Fuchs, N. Groth, and T. Herrling, In vitro and in vivo assessment of the irritation potential of differ- ent spin traps in human skin, Toxicology, 151, 55–63 (2000). (24) Y. Obata, Y. Ashitaka, S. Kikuchi, K. Isowa, and K. Takayama, A statistical approach to the develop- ment of a transdermal delivery system for ondansetron, Int. J. Pharm., 399, 87–93 (2010). (25) R. O. Potts and R.H. Guy, Predicting skin permeability, Pharm. Res., 9, 663–669 (1992). (26) H. A. Benson, Transdermal drug delivery: Penetration enhancement techniques, Curr. Drug. Deliv., 2, 23–33 (2005).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)