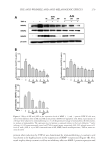
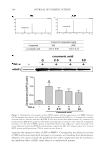
JOURNAL OF COSMETIC SCIENCE 274 in the presence of radio immunoprecipitation assay (RIPA) buffer (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), including protease/phosphatase inhibitor cocktails Sigma). Equal amounts of pro- tein (30 μg) were electrophoresed on 12% SDS-polyacrylamide gels and transferred to a nitrocellulose membrane. Binding of each specifi c antibody was visualized using the enhanced chemiluminescence method (Amersham Biosciences, Pittsburgh, PA). Equal loading of samples was confi rmed by reprobing the membranes with anti-β-actin antibody. The signals were detected by UV transilluminators according to the manufacturer’s specifi ca- tions (DAIHAN Scientifi c Co, Seoul, Korea). The band densities from immunoblots were analyzed using Multi Gauge Ver. 3.0 soft- ware (Fujifi lm, Tokyo, Japan). CELL CYTOTOXICITY ASSAY To evaluate the cytotoxicity of SSE and f SSE, a 3-(4, 5-dimethylthiazol-2-yl)-2, 5- diphenyltetrazolium bromide (MTT) assay was performed as described previously with slight modifi cations (23). Briefl y, HDF-N cells (5 × 103 cells/well) were seeded into 96-well plates and incubated overnight. The cells were then treated with various doses of samples for 24 h. Ten microliters of 5 mg/ml MTT solution were added to each well, and the cells were incubated for 4 h. The formazan crystals were dissolved in DMSO, and the absorbance at 540 nm was determined using a Multireader (TECAN, Infi nite 200, Zürich, Switzerland). Fig ure 1. Effect of 50% ethanol extracted SSE on the expression levels of MMP-1 protein. NIH-3T3 cells were exposed to 50 μg/ml extracts of S. bicolor L. stalk produced using different concentrations of EtOH. Equal amount of cell lysate were subjected to immunoblotting. The level of MMP-1 protein is expressed relative to the level of β-actin. Data are the mean ± SEM (n = 3). * indicate -, control similarity. SSE 50% ethanol extract of S. bicolor L. stalk, f SSE A. oryzae NK–fermented form of SSE, MMP matrix metalloproteinase.
THE ANTI-WRINKLE AND ANTI-MELANOGENIC EFFECTS 275 To exclude the interference of SSE and f SSE in the measurements, wells containing each test agent alone were incubated and reacted with MTT. Absorbance values were normalized to the values obtained for the untreated cells to determine the percentage of survival. ANTIOXIDANT SCAVENGING ACTIVITY The oxygen radical absorbance capacity (ORAC) assay, which measures scavenging activity against peroxy radicals induced by AAPH, was performed as described in a previous report to determine the antioxidant capacities of SSE and f SSE (24). Fluorescein (FL) was used as the fl uorescent probe since the loss of FL fl uorescence is an indication of the extent of damage resulting from its reaction with the peroxy radical. Briefl y, 2 μl of sample or Trolox was incubated with 0.2 mM of β-PE and 200 mM of AAPH in a total volume of 200 μl. To determine decreases in the amount of fl uorescence, fl uorescence was measured at two- minute intervals for 60 min at 37°C. All ORAC analyses were performed on a Synergy HT plate reader at 37°C with an excitation wave length of 535 nm and an emission wave- length of 590 nm. The protective effect of an antioxidant was measured by comparing with a known antioxidant, Trolox, a water-soluble analog of the antioxidative compound, vita- min E. The area under the fl uorescence decay curve (AUC) for FL was calculated as follows: ( ) 1 0 2 0 3 0 19 0 20 0 AUC Areaunderthecurve 1+ f f f f f f f f f f = + + + + "+ ORAC value: sample blank Trolox blank AUC AUC AUC AUC molarityof Trolox molarityof sample ¯ q ¡ ° Final ORAC values were expressed as mean ± SEM. DETERMINATION OF TYROSINASE ACTIVITY Tyrosinase activity was measured using a colorimetric method. Samples were dissolved in DMSO. Each well of the assay plate contained 10 μl of sample with 20 μl of mushroom tyrosinase (1,000 U/ml), and 170 μl of assay mixture (1 mM L-tyrosine, 50 mM phos- phate buffer (PH 6.5), DW= 10:10:9). Reactions were incubated at 25°C for 20 minutes and the absorbance was then measured at 490 nm. Each sample was accompanied by a blank sample that contained all the components except mushroom tyrosinase. The results were compared with a control consisting of DMSO in place of sample. The percentage of tyrosinase activity was calculated as follows: Sample tyrosinse samplealone tyrosinase ¯ q100. ¢ ± HIGH PERFORMANCE LIQUID CHROMATOGRAM ASSAY The Shimadzu LC-20AD HPLC system (Kyoto, Japan) was used with a PDA detector set at 309 nm. The Skypak C18 (250mm × 4.6 mm) was used and temperature was set at 40°C. The gradient for elution consisted of water with 0.5% acetic acid (A) and acetonitrile (B). The
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)