JOURNAL OF COSMETIC SCIENCE 272 skin aging (6). Extrinsic skin aging is caused by environmental factors and results in coarse wrinkles and unevenly distributed pigmentation (7,8). Intrinsic aging is associated with skin damage caused by endogenous reactive oxygen species (ROS). It has been suggested that both aging processes involve oxidative stress and an infl ammatory response in the skin (8,9). In addition to their clinical uses, such as the treatment of pigmentary disorders, skin whitening compounds are used in cosmetics to obtain a lighter skin appearance (2). Because having a youthful appearance may affect mental well-being, body image, and quality of life, skin-care and antiaging cosmetics are the center of attention in different societies in countries around the world, including Asia (10,11). Skin color is determined by a combination of four biochromes, including oxyhaemoglo- bin (red), reduced haemoglobin (blue), carotenoids (yellow), and melanin (orange-black) (3). Melanin is produced in the melanosomes of melanocytes and serves as a shield to protect DNA against damage caused by UV that induces ROS to act as a signal for the proliferation of melanocytes and melanogenesis. However, excessive accumulation of melanin and/or melanosome darkens skin color (12). Melanin production is regulated by a glycoprotein tyrosinase (13). Transcription of tyrosinase is controlled by microphthal- mia transcription factor and/or alpha-melanocyte-stimulating hormone (14). Thus, skin depigmentation can be achieved by interfering before, during, or after melanin synthe- sis. In addititon, the structural integrity of the skin is maintained by extracellular ma- trix, such as Type I collagen, however, collagen breakdown is prevalent in photo-aged skin (5). Matrix metalloproteinases (MMPs) are zinc-containing endopeptidases that play a critical role in the degradation of collagens (15). The expression of MMPs is pri- marily regulated at the transcriptional level, but aberrant activation of transcription factors, such as the nuclear factor kappa B (NF-κB), activator protein-1, signal transduc- ers and activators of transcription, and the Smad family of proteins, causes robust pro- duction of specifi c MMPs (16). Excessive degradation of collagens by MMPs may cause the destruction of healthy skin architecture and result in the formation of wrinkles as- sociated with aging. In a large variety of whitening products, the use of different natural whitening agents is noticeable because of serious safety concerns associated with corti- costeroids, tretinoin, and hydroquinone (2,3). Among botanic ingredients, one of the active ingredients from plants is an arbutin isolated from uva ursi bearberry (3). Numer- ous efforts aimed at preventing skin aging or obtaining skin whitening without safety concerns are underway. Sorghum [Sorghum bicolor (L.) Moench] is a major cereal crop which, along with rice, maize, and wheat, is cultivated all over the world. Sorghum is used in various ways, includ- ing as food, teas, and beer, and its extracts are used in traditional medicine (17). However, unlike the grain, the byproducts of sorghum, such as the stalk, are not actively used. Leaves and stems remaining after grain harvest are used as hay and silage. Recent studies have shown that the stalk of sorghum has antioxidant, anti-infl ammatory, and antianemic properties (18), but its benefi cial effect on skin whitening and its skin antiaging potential have not been studied. The development of safe yet effective melanogenesis inhibitors is one of the challenges of dermatological research and the cosmetic industry. Thus, we investigated the antiwrinkle and antimelanogenesis potentials of an EtOH extract from Sorghum [Sorghum bicolor (L.) Moench] stalk and its fermented Sorghum stalk. We evaluated its in vitro antioxidant capacity, antityrosinase activity, and ability to suppress MMP-1, -2, and -3.
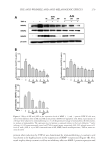
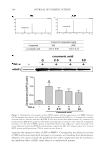
THE ANTI-WRINKLE AND ANTI-MELANOGENIC EFFECTS 273 MATERIAL AND METHODS CHEMICALS AND REAGENTS Water and acetonitrile were high-performance liquid chromatography (HPLC) grade and obtained from Honeywell Burdick & Jackson (Morris Plains, NJ). Arbutin, 6-hydroxy-2,5,7,8- tetramethylchroman-2-carboxylic acid (Trolox®), 2,2′-azobis (2-amidinopropane) dihy- drochloride (AAPH), β-phycoerythrin (β-PE), mushroom tyrosinase, L-tyrosine, p-coumaric acid, and tumor necrosis factor-α (TNF-α) were purchased from Sigma (St. Louis, MO). Unless indicated otherwise, all other chemicals were obtained from Sigma. All antibodies were obtained from Cell Signaling Technologies (Beverly, MA). CELL CULTURE NIH-3T3 cells and a melanoma cell line (B16F10) were obtained from the Korean cell line bank (Seoul, Korea) and were maintained in Dulbecco’s modifi ed Eagle’s medium (Gibco, Grand Island, NY) containing 10% fetal bovine serum (Gibco) and 1% penicillin (Gibco). Melanoma and fi broblast cells were cultured at 37°C in a humidifi ed atmosphere with 5% CO2. HDF-N cell is a primary human neonatal foreskin cell line and widely used for the wrinkle-repair effi cacy test (19). HDF-N cells were obtained from the Amer- ican Type Culture Collection (ATCC, Manassas, VA) and were maintained in FGM-2 Bullet Kits (Lonza, Switzerland) with 1% penicillin (Gibco) at 37°C in a humidifi ed atmosphere with 5% CO2. For the fermentation of Sorghum bicolor L. stalk (SSE), Aspergillus oryzae (A. oryzae) NK, a widely used fi lamentous fungi in the fermentation process containing abundant digestive enzymes (20), was obtained from Seoul Pharmaceutical (Seoul, Korea) and was cultured at 37°C in Potato Dextrose Broth (BD Biosciences, San Jose, CA) which is used for cultivating yeasts and molds in a shaking incubator. PREPARATION OF THE SORGHUM BICOLOR L. STALK EXTRACT AND ITS FERMENTATION Sorghum bicolor L. Moench stalks were purchased from Danjoungbio (WonJoo, Korea), cut into pieces before their use, and dried in the shade at room temperature. The ethanolic extracts of SSE were extracted for 24 h at room temperature using various concentrations of ethanol ranging from 0 to 100%. Each EtOH extract was then lyophilized and stored for use in subsequent experiments. It has been suggested that the suppressive effect of com- pounds or extracts on MMP-1 protein expression is a good indicator of promising candi- dates (21). NIH-3T3 cells were treated for 24 h with extracts from different concentrations of EtOH, and the cell lysates were then subjected to immunoblotting. Based on the sup- pression of MMP-1 expression (Figure 1), a 50% ethanol extract of SSE was chosen. After the addition of 0.5% A. oryzae NK in 50% SSE, fermentation was carried out at 37°C for 48 h using spinning. The fermented SSE was lyophilized and used in subsequent experi- ments after dissolving in dimethyl sulfoxide (DMSO) at the appropriate concentration. IMMUNOBLOT ANALYSIS Protein extraction and immunoblot were performed as previously described (22). Briefl y, cells were washed twice with cold Dulbecco’s phosphate-buffered saline and then homogenized
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)