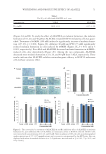
WHITENING AND PROTECTIVE EFFECT OF ALACELL 67 modified Eagle’s medium (DMEM) were supplied by Gibco BRL (Grand Island, NY). α-melanocyte–stimulating hormone (α-MSH), arbutin, dimethyl sulfoxide (DMSO), tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), propylthiouracil (PTU), and phenylmethanesulfonyl fl uoride (PMSF) were purchased from Sigma-Aldrich (St. Louis, MO). SYNTHESIS AN D PURIFICATION OF ALACELL The peptides of the tyrosine–glycine–phenylalanine–leucine (Y-G-G-F-L) were produced according to a chemical synthesis method known in the relevant fi eld of technology (19). ALACELL was synthesized by combining 5-ALA with Y-G-G-F-L peptides. ALACELL was synthesized by solid-phase peptide synthesis techniques, and then purifi ed by RP-HPLC C18 column (Bondapark 5 μm, 250 × 4.6 mm, Waters, Ireland) in a gradient of acetonitrile in 0.1% trifl uoroacetic acid at 215 nm. The peptide moiety Y-G-G-F-L was prepared with a standard solid-phase peptide synthesis method. A matrix-assisted laser desorption ionization time of fl ight mass spectroscopy (MALDI-TOF MS) assay (linear mode, α-cyano-4-hydroxy-cinnamic acid matrix) was performed to ensure the synthetic quality of ALACELL (molecular weight and chemical structure). The MALDI-TOF MS assay (linear mode, α-cyano-4-hydroxy-cinnamic acid matrix) was performed to ensure the syn- thetic quality of ALACELL (molecular weight and chemical structure). A MALDI-TOF MS instrument was used, along with an Axima curved-fi eld refl ectron (Shimadzu/Kratos, Manchester, United Kingdom) instrument, in which gauge pressure was set to 8.0 × 10-4 Pascal gauge pressure, linear mode, and 96-square well sample plate. MALDI-MS data were acquired using an AXIMA-CFR™ Plus-mass spectrometer (Shimadzu Biotech, Japan) in positive ion refl ectron mode. MICRO-ORGANISMS AND CUL TURE C. acnes was obtained f rom the Korean Culture Center of Microorganisms (KCCM, Seoul, South Korea). C. acnes was incubated in Gifu Anaerobic Medium (GAM) for 48 h at 37°C. S. aureus, B. cereus, E. coli, and Y. enterocolitica were obtained from the Chungcheongnam-Do Health and Environment Research Institute in Korea and were incubated for 48 h under aerobic conditions with brain heart infusion (BHI) broth (Difco, Lawrence, MA). DETERMINATION OF ANTIMIC ROBIAL ACTIVITY C. acnes was prepared by incubation in GAM broth for 48 h. S. aureus, B. cereus, E. coli, and Y. enterocolitica were prepared by incubation in BHI broth for 48 h. The concentration of microbial strains was adjusted to 0.5 OD620 nm. A diluted microbial suspension (100 μL) was inoculated into a 96-well microplate. The 50% inhibitory concentration (IC50) was determined in mM for the 5-ALA and ALACELL using a twofold serial dilution assay. Each compound was diluted by DMSO to a concentration of 1,000 μM, and serial dilu- tions were conducted to make a range from 11.90 to 95.33 mM and from 2.33 to 18.70 mM, respectively. A diluted compound (100 μL) was added to a 96-well microplate. A medium blank containing the selective broth and the compound solution was also made for the
JOURNAL OF COSMETIC SCIENCE 68 controls. After incubation for 48 h, the absorbance was measured at 620 nm with a micro- plate reader (EZ read 400, Biochrom Ltd., Cambridge, UK). The results were transformed to a percentage of the controls. The IC50 measure was graphically obtained from the dose– response curves. CELL CULTURE AND CYTOTOXICI TY B16F10 melanoma cells and H aCaT keratinocyte cells that were purchased from the American Type Culture Collection (ATCC, Manassas, VA) were cultured in a 5% CO2 incubator at 37°C. The cells were cultured in DMEM supplemented with 10% FBS and 0.01% antibiotic-antimycotic solution (Invitrogen, Grand Island, NY) in a 5% CO2 incuba- tor at 37°C. The cytotoxic effect of 5-AL A and ALACELL was measured by the MTT assay (20). Briefl y, cells were seeded uniformly at 2.5 × 104 cells/well densities in 96-well micro- plates. After 24 h, the media were replaced with 100 μL media containing fi nal concen- trations equivalent to 10, 20, and 40 μM in 5-ALA and ALACELL. The MTT assay was performed after 48 h of incubation with 5-ALA and ALACELL. The cytotoxicity was quantifi ed by measuring UV absorbance at 570 nm by a Tecan microplate reader (Mannedorf, Switzerland). The measured absorbance was standardized to the absorbance of nontreated control cells. All data represent the mean and standard deviation from at least three separate experiments and were compared using a student’s t-test. EFFECT OF ALACELL ON MELANIN FORM ATION The melanin formation was analyze d by a modifi cation of the method described by a previous study (21). B16F10 melanoma cells were seeded at a density of 3 × 105 cells/well in 96-well microplates and cultivated by the method described earlier. To induce hyper- production of melanin, the cells were treated with α-MSH of 100 nM. Then, the concen- trations of 10, 20, and 40 μM of 5-ALA and ALACELL were added to the medium and further incubated for 48 h. To remove melanin excreted from the cells, the medium was removed, and the cells were washed twice with PBS, and then harvested by trypsin treat- ment. The harvested cells were pelleted, and the cell membrane was dissolved in Triton X-100 (Sigma-Adrich, St. Louis, MO). The purifi ed melanin was dissolved in 2 M NaOH for 30 min at 100°C. The absorbance was measured at 405 nm. The melanin content was compared with untreated control cells. EFFECT OF ALACELL ON INTRACELLULAR TY ROSINASE ACTIVITY Intracellular tyrosinase activity can rapidly oxidize L-tyrosine to L-3,4-dihydroxyphenyl- alanine (L-DOPA) and further convert it to dopaquinone, and then the activity of tyrosi- nase determines the amount of brown dopaquinone (22,23). In this study, B16F10 melanoma cells were seeded at a density of 3 × 105 cells/well in 96-well microplates, and the culture medium was discarded after 24 h. Five-ALA and ALACELL were treated with 10, 20, and 40 μM. After 48 h, the cells were washed twice with PBS. B16F10 melanoma cells were lysed in 1% Triton X-100, 20 mM sodium phosphate (pH 6.8), and 1 mM
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)