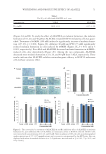
ANTIAGING POTENTIAL OF FUCOXANTHIN 55 anti-infl ammatory activities (21). Fucoxanthin has various physiological activities such as anticancer, anti-obesity, antiangiogenic, and neuroprotective activities (18,23–26). The aim of this study was to investigate the antiaging effect of fucoxanthin concentrate derived from Phaeodactylum tricornutum and evaluate its potential use in wrinkle care cosmetics. MATER IAL AND METHODS MATER IALS Phaeo dactylum tricornutum concentrate containing ≥50% fucoxanthin (PT-FX50) was pro- vided by Systems Biotechnology Research Center of the Korea Institute of Science and Technology (Gangneung, Korea) (27). High-performance liquid chromatography (HPLC)–mass spectrometry and HPLC–photodiode array detection (PDA) were used to assay the amount of fucoxanthin in P. tricornutum concentrate powder qualitatively and quantitatively. The fucoxanthin content in the powder was found to be 51.5%. In addition, P. tricornutum concentrate was diluted to 2% with caprylic/capric triglycerides (PT-FX01) and used as the fucoxanthin raw material to prepare a wrinkle care cream. The concentra- tion of fucoxanthin in PT-FX01 was determined to be 1.01% by HPLC-PDA. CELL V IABILITY ASSAY Cell v iability was measured using EZ-Cytox enhanced cell viability kit (Daeil Lab Ser- vice, Seoul, Korea) according to the manufacturer’s instructions. In brief, human dermal fi broblast, neonatal (HDFN) cells were cultured in Dulbecco’s modifi ed Eagle medium (DMEM) containing 10% fetal bovine serum, 1% penicillin/streptomycin, and 4 mM L-glutamine at 37°C in a humidifi ed atmosphere of 5% CO2. The cells were seeded at a density of 1 × 104 cells/well in a 96-well plate and cultured for 24 h. PT-FX50 was dis- solved in dimethyl sulfoxide to prepare a 10% stock solution, which was serially diluted with DMEM and added to the culture medium to treat with 3.125, 6.25, 12.5, 25, 50, and 100 ppm of PT-FX50. Cell viability was determined 24 h later. The EZ-Cytox reac- tion mixture (Ez-3000, Daeil Lab Service) was diluted 10-fold by mixing it with the medium. Next, 100 μL of the diluted reaction mixture was added to each well. After 1 h of incubation, cell viability was determined by measuring absorbance at 450 nm and expressing it as a percentage relative to the viability of untreated cells. PROCOLLAG EN, MATRIX METALLOPROTEINASE (MMP)-1, MMP-2, AND MMP-9 ASSAYS HDFN cell s were seeded at a density of 1 × 104 cells/well in a 96-well plate and cultured for 24 h. The medium was removed and kept for 24 h in a starvation state. Next, the stock PT-FX50 solution was diluted with DMEM to different concentrations, after which the cells were treated and cultured for 24 h. The culture medium was harvested, and the amount of procollagen in it was measured using procollagen type I c-peptide EIA kit (Mk1010 Takara, Shiga, Japan). 10 ng/ml of transforming growth factor (TGF)-β was used as a positive control for procollagen synthesis assay. In addition, the amounts of
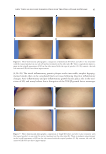
JOURNAL OF COSMETIC SCIENCE 56 MMP-1, MMP-2, and MMP-9 in the culture medium were measured using human total MMP-1 (DY901), MMP-2 (DY902), and MMP-9 (DY911) ELISA kits (R&D systems, Minneapolis, MN), respectively. As a positive control of MMP expression analysis, 10 μM RA, well known as MMP inhibitor and antiaging agent, was used (28). The expression levels of procollagen and MMPs were quantifi ed based on the amount of protein in the cells. Cells attached to the bottom of the plate were washed with Dulbecco’s phosphate- buffered saline and lysed with 1 N NaOH, after which the amount of protein was mea- sured using a bicinchoninic acid (BCA) assay method (BCA assay kit, #23209 Pierce, Hercules, CA). FORMULATION OF WRINKLE CARE CREAM The cream wa s prepared with water, PT-FX01 (fucoxanthin and caprylic/capric triglycer- ide), PEG/PPG-17/6 copolymer, glycerin, butylene glycol, trehalose, hydrolyzed colla- gen, cetearyl alcohol, arachidyl alcohol, behenyl alcohol, arachidyl glucoside, glyceryl stearate, PEG-100 stearate, canola oil, catearylethyl hexanoate, shea butter, carbomer, tromethamine, disodium ethylenediaminetetraacetic acid, and fragrance. All the ingredi- ents were of cosmetic grade. The concentration of fucoxanthin in cream was 150 mg/kg. A placebo cream was similarly prepared without PT-FX01. RECRUITMENT OF VOLUNTEERS Twenty-one K orean women (age: mean, 44.2 ± 5.36 years range, 31–55 years) with wrinkles around their eyes were recruited for the study. The control and test products were distributed to the subjects to be used consecutively for 8 weeks. Participants were instructed to apply the test product on one side of the face and the same amount of the control sample on the opposite side. The subjects voluntarily participated in the study, and it was ensured that they had no skin disease. The rights, safety, and welfare of the subjects were protected. The study was performed in accordance with the Helsinki Dec- laration and Good Clinical Practice Guidelines. Subjects visited the test room three times during the study as follows: before using the samples and at 4 and 8 weeks after the fi rst use. The temp erature and humidity in the test room were 22 ± 2°C and 50% ± 5%, re- spectively. The subjects were acclimated to the test room conditions for at least 30 min before measurements were taken. MEASUREMENT OF S KIN MOISTURE The hydration le vel at the skin surface (stratum corneum) was evaluated by measuring electrical capacitance with Corneometer® CM 825 (Courage + Khazaka GmbH, Cologne, Germany). A probe with a diameter of 10 mm was placed in contact with the skin of the cheek and pressed with a force of 1.1–1.5 N to take measurements. Each result was ex- pressed as an arbitrary unit value between 1 and 130. Larger values were indicative of a high moisture content. Each measurement was repeated three times, and the average value was calculated.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)