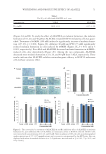
WHITENING AND PROTECTIVE EFFECT OF ALACELL 69 PMSF, and centrifuged at 14,000 rpm for 15 min. The Bradford assay with bovine serum albumin as the protein standard was conducted to measure the protein content of the supernatant. Intracellular tyrosinase activity was measured in a reaction mixture (1 mL) containing 50 mM phosphate buffer (pH 6.8), 2 mM L-DOPA, and 300 μg supernatant proteins. After incubation at 37°C for 15 min, the absorbance was measured at 475 nm by a microplate reader. Tyrosinase activity (%) = [(A-B)/C] × 100% A: sample absor- bance volume, B: blank absorbance volume, and C: control absorbance volume. EFFECT OF ALACELL ON UVB-IRRADIATED HaCaT CELL S UVB irradiation was performed by UVM-225D Mine ralight UV display lamps (UVP, Phoenix, AZ) generating UVB light in the range of 290–320 nm with a maximum emis- sion wavelength of 302 nm. UV doses were measured using a HD2102-2 UV meter (Delta OHM, Padova, Italy). HaCaT keratinocyte cells were seeded on six-well plates at 7 × 104 cells/well and incubated in a 5% CO2 incubator at 37°C. Five-ALA and ALACELL were treated in cultured cells at concentrations of 20, 40, 80, and 100 μM, and after 1 h, the cells were irradiated with UVB at 40, 80, and 120 mJ/cm2. To prevent UV quenching, before irradiation, the cell culture medium was replaced by the same volume of PBS after two washing steps with PBS. After UVB irradiation, cells were fed with fresh growth medium and incubated. After 24 h, the cell viability was measured by the MTT assay described earlier. STATISTICAL ANALYSIS Results are expressed as mean s ± standard deviatio n. Data were analyzed using analysis of variance and Duncan’s multiple range tests. Signifi cance was indicated at *p 0.05, **p 0.01, and ***p 0.001. RESULTS CONFIRMATION OF ALACELL STRUCTURE BY MALDI-T OF MS AL ACELL was synthesized by combining the phytochemic al agent 5-ALA with Y-G-G- F-L peptide to support physicochemical activity. MALDI-TOF mass spectrometry was performed to identify the molecular weight and chemical structure of ALACELL (Figure 1A). The chemical formula of ALACELL is C33H44N6O9, and its molecular weight is 668.32 (Figure 1B). GROWTH INHIBITORY EFFECT OF ALACELL AGAINST C. ACNES To investigate the antimicrobial activities of 5-ALA and ALACELL, we determined the minimum concentration that produces 50% inhibition of bacterial growth (IC50) by the broth dilution assay. We tested different concentrations for each sample against C. acnes, S. aureus, B. cereus, E. coli, and Y. enterocolitica. 5-ALA and ALACELL against C. acnes blocked growth dose dependently, respectively (Table I). The IC50 of 5-ALA and ALACELL against
JOURNAL OF COSMETIC SCIENCE 70 C. acnes were identifi ed as 18.01 and 6.12 mM, respectively (Table I). The inhibitory effect of ALACELL against C. acnes was higher than that of 5-ALA. However, antimicrobial activ- ity against S. aureus, B. cereus, E. coli, and Y. enterocolitica did not appear (data not shown). ALACELL DECREASED MELANIN FORMATION WITHOUT CYTOTOXI CITY IN B16F10 CELLS The effect of 5-ALA and ALACELL on B16F10 melanoma c ell proliferation showed that both compounds did not have signifi cant cytotoxicity against the concentration test Figure 1. Chemical structure of ALACELL (A) and analysis by MALDI-TOF mass spectrometry (B). ALACELL was synthesized by combining 5-ALA with Y-G-G-F-L peptide. After purifying by reverse-phase HPLC of ALACELL, a MALDI-TOF mass spectrometry was performed and MALDI-MS data were analyzed by u sing AximaCFR™ Plus-mass spectrometer in positive ion refl ectron mode.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)