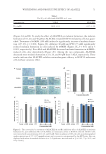
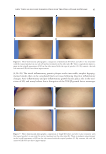
NEW TOPICAL SILICONE FORMULATION FOR TREATING STRIAE DISTENSAE 81 improvement, and 13.6% had no change in texture. After 1 mo of no treatment, 100.0% of SD were reported as having no change in texture (Table IV). To lerability. Th e topical product was reported to be tolerated well and with no issues in 90.9% of cases (Table V). Overall, 9.1% of the treated group reported mild issues such as slight itching or redness the fi rst day of application, which subsided by day 2 in all patients. In all, 86.4% of SD treated with the topical product were rated as having better overall appearance and texture by patients (Figure 3) and 0.0% of untreated SD were rated as having better overall appearance and texture. This was verifi ed to be statistically signifi - cant by the Wilcoxon signed-rank test. Overall, 90.9% of SD treated with the topical product and untreated SD were rated the same for favorable tolerability to treatment and 9.1% of untreated SD had better ratings for tolerability to treatment. However, a Wilcoxon signed-rank test found there to be no statistically signifi cant differences between SD treated with the topical product and untreated SD in response to tolerability to treatment. And, 20 of 22 patients (90.9%) indicated they would continue to use the topical product after the 1-mo duration of the study. Figure 2. Patient reported o verall appearance: Average distribution of scores. Table IV Patient-Reported Outcome, Average Scores for Texture Treatment Score Proportion (%) N = 22 patients 95% margin of error Topical product No change 3/22 (13.6%) ±14.3% Mild improvement 11/22 (50.0%) ±20.9% Moderate improvement 4/22 (18.2%) ±16.1% Signifi cant improvement 4/22 (18.2%) ±16.1% No treatment No change 22/22 (100.0%) ±0.0% Mild improvement 0/22 (0.0%) ±0.0% Moderate improvement 0/22 (0.0%) ±0.0% Signifi cant improvement 0/22 (0.0%) ±0.0%
JOURNAL OF COSMETIC SCIENCE 82 IN DEPENDENT EVALUATOR DATA Ov erall appearance. Af ter 1 mo of using the topical product, 29.5% of SD had shown mild improvement, 34.1% had shown moderate improvement, 9.1% had shown sig- nifi cant improvement, 27.3% had no change in overall appearance, and none of the SD worsened. After 1 mo of no treatment, 29.5% of SD had shown mild improvement, 6.8% had shown moderate improvement, 61.4% had shown no change, 2.3% had worsened in overall appearance, and none had shown signifi cant improvement (Table VI, Figure 4). On average, 86.4% of SD that were treated with the topical product were rated as having better overall appearance by independent evaluators. On average, 0.0% of untreated SD Table V Patient-Reported Outcome, Average Scores for Tolerability Treatment Score (count), N = 22 patients Proportion (%), N = 22 patients 95% margin of error Topical product No issues 20/22 (90.9%) ±12.0% Mild issues 2/22 (9.1%) ±12.0% Moderate issues 0/22 (0.0%) ±0.0% Severe intolerance 0/22 (0.0%) ±0.0% No treatment No issues 22/22 (100.0%) ±0.0% Mild issues 0/22 (0.0%) ±0.0% Moderate issues 0/22 (0.0%) ±0.0% Severe intolerance 0/22 (0.0%) ±0.0% Figure 3. Patient reported a v erage scores for overall appearance of topically treated SD.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)