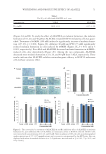
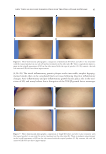
NEW TOPICAL SILICONE FORMULATION FOR TREATING STRIAE DISTENSAE 79 were also excluded from the study because of possible compounding factors in SD. Patients with SD alba were included in the study, and those with SD rubra or purpuric were excluded. In this split study, participants were assigned based on a computer-generated randomization protocol such that half, laterally, of the subject’s SD were treated with the proprietary product and half were left untreated. Participants were instructed to massage the product into the designated SD two times per day for 1 mo. Subjects were assessed at baseline and again at 1 mo. At the month 1 follow-up visit, patient-reported outcome surveys were obtained for each side. Subjects were asked to grade the overall appearance and texture of their SD as no change, mild, moderate, or signifi cant improvement through a survey. Subjects were also asked to grade their toler- ability of the product as no, mild, moderate, or severe issues (Table I). Last, subjects were asked if they would continue with this treatment, yes or no. Three-dimensional imaging was performed before treatment was administered and at the 1-mo follow-up visit using a standardized Canfi eld Vectra 3D imaging system (Canfi eld Scientifi c Inc., Parsippany, NJ). Two independent evaluators, both licensed physicians in their residency training (dermatologist and plastic and reconstructive surgeon), blinded to the study treatment, assessed the images before and after treatment and graded the appearance of each side on overall appearance: –1 indicating worsened appearance, 0 indicating no change, 1 indi- cating mild improvement, 2 indicating moderate improvement, and 3 indicating sig- nifi cant improvement (Table II, Figure 1). In addition, the evaluator was also asked to indicate which side appeared better overall, left or right. RESULTS Twenty-two subjects (20 females and two males) met the criteria and were enrolled in this study. This included 11 abdomen, four thigh, and seven buttock SD. All subjects had SD alba. The mean age of subjects was 36.5 (SD ± 9.8) years. Table I Patient-Reported Outcome Score 0 1 2 3 Overall Appearance No change Mild improvement Moderate improvement Signifi cant improvement Texture No change Mild improvement Moderate improvement Signifi cant improvement Tolerability No issues Mild issues Moderate issues Severe issues Table II Independent Evaluator Scale Score -1 0 1 2 3 Overall appearance Worsened No change Mild improvement Moderate improvement Signifi cant improvement
JOURNAL OF COSMETIC SCIENCE 80 PATIENT-REPORTED OUTCOMES Ov erall appearance. Af ter 1 mo of using the topical product, 40.9% of SD had shown mild improvement, 31.8% had shown moderate improvement, 13.6% had shown signifi cant improvement, and 13.6% had no change in overall appearance. After 1 mo of no treat- ment, 100.0% of SD were reported as having no change in overall appearance (Table III, Figure 2). Te xture. Af ter 1 mo of using the topical product, 50.0% of SD had shown mild im- provement, 18.2% had shown moderate improvement, 18.2% had shown signifi cant Figure 1. Independent eval u ator grading scale. Table III Patient-Reported Outcome, Average Scores for Overall Appearance Treatment Score Proportion (%), N = 22 patients 95% margin of error Topical product No change 3/22 (13.6%) ±14.3% Mild improvement 9/22 (40.9%) ±20.5% Moderate improvement 7/22 (31.8%) ±19.5% Signifi cant improvement 3/22 (13.6%) ±14.3% No treatment No change 22/22 (100.0%) ±0.0% Mild improvement 0/22 (0.0%) ±0.0% Moderate improvement 0/22 (0.0%) ±0.0% Signifi cant improvement 0/22 (0.0%) ±0.0%
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)